Gas6 induces growth, beta-catenin stabilization, and T-cell factor transcriptional activation in contact-inhibited C57 mammary cells
- PMID: 11154277
- PMCID: PMC86681
- DOI: 10.1128/MCB.21.3.902-915.2001
Gas6 induces growth, beta-catenin stabilization, and T-cell factor transcriptional activation in contact-inhibited C57 mammary cells
Abstract
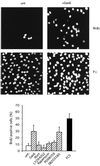
Gas6 is a growth factor related to protein S that was identified as the ligand for the Axl receptor tyrosine kinase (RTK) family. In this study, we show that Gas6 induces a growth response in a cultured mammalian mammary cell line, C57MG. The presence of Gas6 in the medium induces growth after confluence and similarly causes cell cycle reentry of density-inhibited C57MG cells. We show that Axl RTK but not Rse is efficiently activated by Gas6 in density-inhibited C57MG cells. We have analyzed the signaling required for the Gas6 proliferative effect and found a requirement for PI3K-, S6K-, and Ras-activated pathways. We also demonstrate that Gas6 activates Akt and concomitantly inhibits GSK3 activity in a wortmannin-dependent manner. Interestingly, Gas6 induces up-regulation of cytosolic beta-catenin, while membrane-associated beta-catenin remains unaffected. Stabilization of beta-catenin in C57MG cells is correlated with activation of a T-cell factor (TCF)-responsive transcriptional element. We thus provide evidence that Gas6 is mitogenic and induces beta-catenin proto-oncogene stabilization and subsequent TCF/Lef transcriptional activation in a mammary system. These results suggest that Gas6-Axl interaction, through stabilization of beta-catenin, may have a role in mammary development and/or be involved in the progression of mammary tumors.
Figures






References
-
- Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro e in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Allen M P, Zeng C, Schneider K, Xiong X, Meintzer M K, Bellosta P, Basilico C, Varnum B, Heidenreich K A, Wierman M E. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol Endocrinol. 1999;13:191–201. - PubMed
-
- Barker N, Morin P J, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 2000;77:1–24. - PubMed
-
- Barnard D, Sun H, Baker L, Marshall M S. In vitro inhibition of Ras-Raf association by short peptides. Biochem Biophys Res Commun. 1998;247:176–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
