Nodal endoplasmic reticulum, a specialized form of endoplasmic reticulum found in gravity-sensing root tip columella cells
- PMID: 11154334
- PMCID: PMC61007
- DOI: 10.1104/pp.125.1.252
Nodal endoplasmic reticulum, a specialized form of endoplasmic reticulum found in gravity-sensing root tip columella cells
Abstract
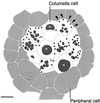
The endoplasmic reticulum (ER) of columella root cap cells has been postulated to play a role in gravity sensing. We have re-examined the ultrastructure of columella cells in tobacco (Nicotiana tabacum) root tips preserved by high-pressure freezing/freeze-substitution techniques to gain more precise information about the organization of the ER in such cells. The most notable findings are: the identification of a specialized form of ER, termed "nodal ER," which is found exclusively in columella cells; the demonstration that the bulk of the ER is organized in the form of a tubular network that is confined to a peripheral layer under the plasma membrane; and the discovery that this ER-rich peripheral region excludes Golgi stacks, vacuoles, and amyloplasts but not mitochondria. Nodal ER domains consist of an approximately 100-nm-diameter central rod composed of oblong subunits to which usually seven sheets of rough ER are attached along their margins. These domains form patches at the interface between the peripheral ER network and the ER-free central region of the cells, and they occupy defined positions within central and flanking columella cells. Over one-half of the nodal ER domains are located along the outer tangential walls of the flanking cells. Cytochalasin D and latrunculin A cause an increase in size and a decrease in numbers of nodal ER domains. We postulate that the nodal ER membranes locally modulate the gravisensing signals produced by the sedimenting amyloplasts, and that the confinement of all ER membranes to the cell periphery serves to enhance the sedimentability of the amyloplasts in the central region of columella cells.
Figures







Similar articles
-
Demonstration of prominent actin filaments in the root columella.Planta. 2001 Feb;212(3):392-403. doi: 10.1007/s004250000406. Planta. 2001. PMID: 11289604
-
Columella cells revisited: novel structures, novel properties, and a novel gravisensing model.Gravit Space Biol Bull. 2000 Jun;13(2):95-100. Gravit Space Biol Bull. 2000. PMID: 11543286
-
Macromolecular differentiation of Golgi stacks in root tips of Arabidopsis and Nicotiana seedlings as visualized in high pressure frozen and freeze-substituted samples.Protoplasma. 1990;157(1-3):75-91. doi: 10.1007/BF01322640. Protoplasma. 1990. PMID: 11537090
-
Cell biology of plant gravity sensing.Adv Space Res. 1994;14(8):117-9. doi: 10.1016/0273-1177(94)90394-8. Adv Space Res. 1994. PMID: 11537908 Review.
-
Complex physiological and molecular processes underlying root gravitropism.Plant Mol Biol. 2002 Jun-Jul;49(3-4):305-17. Plant Mol Biol. 2002. PMID: 12036256 Review.
Cited by
-
Comparative study of cellular structures implicated in gravisensing in statocytes of primary and lateral roots of Vigna angularis.Protoplasma. 2006 Nov;229(1):83-91. doi: 10.1007/s00709-006-0188-9. Epub 2006 Oct 6. Protoplasma. 2006. PMID: 17019525
-
Modifications in Ultrastructural Characteristics and Redox Status of Plants under Environmental Stress: A Review.Plants (Basel). 2023 Apr 16;12(8):1666. doi: 10.3390/plants12081666. Plants (Basel). 2023. PMID: 37111889 Free PMC article. Review.
-
Multiple roles for membrane-associated protein trafficking and signaling in gravitropism.Front Plant Sci. 2012 Dec 11;3:274. doi: 10.3389/fpls.2012.00274. eCollection 2012. Front Plant Sci. 2012. PMID: 23248632 Free PMC article.
-
Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots.Plant Physiol. 2001 Feb;125(2):1045-60. doi: 10.1104/pp.125.2.1045. Plant Physiol. 2001. PMID: 11161060 Free PMC article.
-
A Core Regulatory Pathway Controlling Rice Tiller Angle Mediated by the LAZY1-Dependent Asymmetric Distribution of Auxin.Plant Cell. 2018 Jul;30(7):1461-1475. doi: 10.1105/tpc.18.00063. Epub 2018 Jun 18. Plant Cell. 2018. PMID: 29915152 Free PMC article.
References
-
- Baluska F, Kreibaum A, Vitha S, Parker JS, Barlow PW, Sievers A. Central root cap cells are depleted of endoplasmic microtubules and actin microfilament bundles: implications for their role as gravity-sensing statocytes. Protoplasma. 1997;196:212–223. - PubMed
-
- Barlow PW. The root cap. In: Torrey J, Clarkson DT, editors. The Development and Function of Roots. London: Academic Press; 1975. pp. 21–54.
-
- Barlow PW, Hawes CR, Horne JC. Structure of amyloplasts and endoplasmic reticulum in the root caps of Lepidium sativum and Zea mays observed after selective membrane staining and by high-voltage electron microscopy. Planta. 1984;160:363–371. - PubMed
-
- Buckley IK. Studies in fixation for electron microscopy using cultures cells. Lab Invest. 1973;29:398–410. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

