Identification of the maize amyloplast stromal 112-kD protein as a plastidic starch phosphorylase
- PMID: 11154342
- PMCID: PMC61015
- DOI: 10.1104/pp.125.1.351
Identification of the maize amyloplast stromal 112-kD protein as a plastidic starch phosphorylase
Abstract
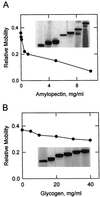
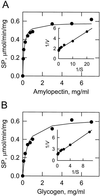
Amyloplast is the site of starch synthesis in the storage tissue of maize (Zea mays). The amyloplast stroma contains an enriched group of proteins when compared with the whole endosperm. Proteins with molecular masses of 76 and 85 kD have been identified as starch synthase I and starch branching enzyme IIb, respectively. A 112-kD protein was isolated from the stromal fraction by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to tryptic digestion and amino acid sequence analysis. Three peptide sequences showed high identity to plastidic forms of starch phosphorylase (SP) from sweet potato, potato, and spinach. SP activity was identified in the amyloplast stromal fraction and was enriched 4-fold when compared with the activity in the whole endosperm fraction. Native and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analyses showed that SP activity was associated with the amyloplast stromal 112-kD protein. In addition, antibodies raised against the potato plastidic SP recognized the amyloplast stromal 112-kD protein. The amyloplast stromal 112-kD SP was expressed in whole endosperm isolated from maize harvested 9 to 24 d after pollination. Results of affinity electrophoresis and enzyme kinetic analyses showed that the amyloplast stromal 112-kD SP preferred amylopectin over glycogen as a substrate in the synthetic reaction. The maize shrunken-4 mutant had reduced SP activity due to a decrease of the amyloplast stromal 112-kD enzyme.
Figures



References
-
- Akatsuka T, Nelson OE. Starch granule-bound adenosine diphosphate Glc-starch glucosyltransferases of maize seeds. J Biol Chem. 1966;241:2280–2286. - PubMed
-
- Ball S, Guan H, James M, Myers A, Keeling P, Mouille G, Buleon A, Colonna P, Preiss J. From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell. 1996;86:349–352. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principles of protein dye-binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Burr B, Nelson OE. The phosphorylases of developing maize seeds. Ann NY Acad Sci. 1973;210:129–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

