Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean
- PMID: 11154344
- PMCID: PMC61017
- DOI: 10.1104/pp.125.1.369
Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean
Abstract
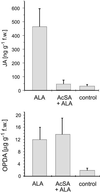
Alamethicin (ALA), a voltage-gated, ion channel-forming peptide mixture from Trichoderma viride, is a potent elicitor of the biosynthesis of volatile compounds in lima bean (Phaseolus lunatus). Unlike elicitation with jasmonic acid or herbivore damage, the blend of substances emitted comprises only the two homoterpenes, 4,11-dimethylnona-1,3,7-triene and 4,8,12-trimethyltrideca-1,3,7,11-tetraene, and methyl salicylate. Inhibition of octadecanoid signaling by aristolochic acid and phenidone as well as mass spectrometric analysis of endogenous jasmonate demonstrate that ALA induces the biosynthesis of volatile compounds principally via the octadecanoid-signaling pathway (20-fold increase of jasmonic acid). ALA also up-regulates salicylate biosynthesis, and the time course of the production of endogenous salicylate correlates well with the appearance of the methyl ester in the gas phase. The massive up-regulation of the SA-pathway (90-fold) interferes with steps in the biosynthetic pathway downstream of 12-oxophytodienoic acid and thereby reduces the pattern of emitted volatiles to compounds previously shown to be induced by early octadecanoids. ALA also induces tendril coiling in various species like Pisum, Lathyrus, and Bryonia, but the response appears to be independent from octadecanoid biosynthesis, because inhibitors of lipoxygenase and phospholipase A(2) do not prevent the coiling reaction.
Figures


References
-
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An elicitor of plant volatiles from beet armyworm oral secretions. Science. 1997;276:945–949.
-
- Blechert S, Bockelmann M, Füβlein M, von Schrader T, Stelmach B, Niesel U, Weiler EW. Structure-activity analyses reveal the existence of two separate groups of active octadecanoids in elicitation of the tendril-coiling response of Bryonia dioica Jacq. Planta. 1999;207:470–479.
-
- Boland W, Gäbler A, Gilbert M, Feng Z. Biosynthesis of C11 and C16 homoterpenes in higher plants: stereochemistry of the C-C-bond cleavage reaction. Tetrahedron. 1998;54:14725–14736.
-
- Boland W, Hopke J, Donath J, Nüske F, Bublitz F. Jasmonic acid and coronatine induce volatile biosynthesis in plants. Angew Chem Int Ed Engl. 1995;34:1600–1602.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

