Impaired vasodilator response to organic nitrates in isolated basilar arteries
- PMID: 11156558
- PMCID: PMC1572527
- DOI: 10.1038/sj.bjp.0703768
Impaired vasodilator response to organic nitrates in isolated basilar arteries
Abstract
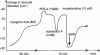
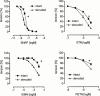
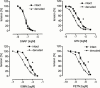
1. The differential responsiveness of various sections and regions in the vascular system to the vasodilator activity of organic nitrates is important for the beneficial antiischaemic effects of these drugs. In this study we examined the vasodilator activity of organic nitrates in cerebral arteries, where vasodilation causes substantial nitrate induced headache. 2. Isolated porcine basilar and coronary arteries were subjected to increasing concentrations of glyceryl trinitrate (GTN), isosorbide-5-nitrate (ISMN) and pentaerythritol tetranitrate (PETN). S-nitroso-N-acetyl-D,L-penicillamine (SNAP) and endothelium-dependent vasodilation was investigated for comparison purpose. 3. The vasodilator potency (halfmaximal effective concentration in -logM) of GTN (4.33+/-0.1, n=8), ISMN (1.61+/-0.07, n=7) and PETN (>10 microM, n=7) in basilar arteries was more than 100 fold lower than that of GTN (6.52+/-0.06, n=12), ISMN (3.66+/-0.08, n=10) and PETN (6.3+/-0.13, n=8) observed in coronary arteries. 4. In striking contrast, the vasodilator potency of SNAP (halfmaximal effective concentration in -logM) was almost similar in basilar (7.76+/-0.05, n=7) and coronary arteries (7.59+/-0.05, n=9). Likewise, no difference in endothelium dependent relaxation was observed. 5. Denudation of the endothelium resulted in a small increase of the vasodilator potency (halfmaximal effective concentration in -logM) of GTN (4.84+/-0.09, n=7, P<0.03) in basilar arteries and similar results were obtained in the presence of the NO-synthase inhibitor N(omega)-nitro-L-arginine (4.59+/-0.05, n=9, P<0.03). 6. These results suggest that cerebral conductance blood vessels such as porcine basilar arteries seems to have a reduced expression and/or activity of certain cellular enzymatic electron transport systems such as cytochrome P450 enzymes, which are necessary to bioconvert organic nitrates to NO.
Figures

References
-
- AHLNER J., ANDERSON R.G.G., TORFGÅRD K., AXELSSON K.L. Organic nitrate esters: Clinical use and mechanisms of actions. Pharmacol. Rev. 1991;43:351–423. - PubMed
-
- BASSENGE E., STUART D.J. Effects of nitrates in various vascular sections and regions. Z. Kardiol. 1986;75 Suppl 3:1–7. - PubMed
-
- BENNETT B.M., MCDONALD B.J., NIGAM R., LONG P.G., SIMON W.C. Inhibition of nitrovasodilator- and acetylcholine-induced relaxation and cyclic GMP accumulation by the cytochrome P-450 substrate, 7-ethoxyresorufin. Can. J. Physiol. Pharmacol. 1992;70:1297–1303. - PubMed
-
- CHUNG S.-J., FUNG H.-L. Identification of the subcellular site for nitroglycerin metabolism to nitric oxide in bovine coronary smooth muscle cells. J. Pharmacol. Exp. Ther. 1990;253:614–619. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

