Pharmacokinetic-pharmacodynamic modelling in the early development phase of anti-psychotics: a comparison of the effects of clozapine, S 16924 and S 18327 in the EEG model in rats
- PMID: 11156572
- PMCID: PMC1572549
- DOI: 10.1038/sj.bjp.0703791
Pharmacokinetic-pharmacodynamic modelling in the early development phase of anti-psychotics: a comparison of the effects of clozapine, S 16924 and S 18327 in the EEG model in rats
Abstract
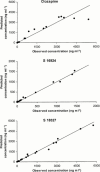
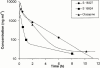
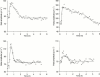
1. The use of pharmacokinetic/pharmacodynamic (PK/PD) analysis in early compound development was investigated in the rat for two developmental anti-psychotic compounds with clozapine as a positive control. 2. Three plasma samples were collected from each of eight animals according to a pre-defined sampling matrix allowing a total of 12 time points for PK analysis. Quantitative electroencephalography (QEEG), particularly the theta and beta frequencies, was used as a measurement of pharmacological effect. 3. PK/KD modelling of the sparse PK data available relative to a rich set of PD data was achieved using a population approach in NONMEM (IV). Individual PK parameter estimates were incorporated into a PK/PD model. 4. Qualitative EEG changes in rat and human were similar for clozapine, but different for the two developmental compounds, suggesting that changes in these PD parameters may not be specifically related to the anti-psychotic activity. 5. Although no definitive data are available concerning the signal specificity of EEG frequency bands with respect to dopaminergic or serotonergic receptor activity, qualitative and quantitative differences seen in EEG parameters are likely to result from the multiple receptor occupancy for these compounds. 6. The results confirm the value of population PK/PD modelling in conjunction with sparse sampling to enable determination of concentration effect relationships in the pre-clinical development programme of CNS-active drugs.
Figures
Similar articles
-
Population pharmacokinetic/pharmacodynamic model of clozapine for characterizing the relationship between accumulated exposure and PANSS scores in patients with schizophrenia.Ther Drug Monit. 2014 Jun;36(3):378-86. doi: 10.1097/FTD.0000000000000014. Ther Drug Monit. 2014. PMID: 24342896
-
S18327 (1-[2-[4-(6-fluoro-1, 2-benzisoxazol-3-yl)piperid-1-yl]ethyl]3-phenyl imidazolin-2-one), a novel, potential antipsychotic displaying marked antagonist properties at alpha(1)- and alpha(2)-adrenergic receptors: II. Functional profile and a multiparametric comparison with haloperidol, clozapine, and 11 other antipsychotic agents.J Pharmacol Exp Ther. 2000 Jan;292(1):54-66. J Pharmacol Exp Ther. 2000. PMID: 10604931
-
Pharmacokinetic-pharmacodynamic characterization of the cardiovascular, hypnotic, EEG and ventilatory responses to dexmedetomidine in the rat.J Pharmacol Exp Ther. 1997 Dec;283(3):1051-8. J Pharmacol Exp Ther. 1997. PMID: 9399976
-
PK-PD integration and PK-PD modelling of nonsteroidal anti-inflammatory drugs: principles and applications in veterinary pharmacology.J Vet Pharmacol Ther. 2004 Dec;27(6):491-502. doi: 10.1111/j.1365-2885.2004.00618.x. J Vet Pharmacol Ther. 2004. PMID: 15601443 Review.
-
Integration and modelling of pharmacokinetic and pharmacodynamic data to optimize dosage regimens in veterinary medicine.J Vet Pharmacol Ther. 2004 Dec;27(6):467-77. doi: 10.1111/j.1365-2885.2004.00613.x. J Vet Pharmacol Ther. 2004. PMID: 15601441 Review.
Cited by
-
Dopamine D2 receptor occupancy as a predictor of catalepsy in rats: a pharmacokinetic-pharmacodynamic modeling approach.Pharm Res. 2014 Oct;31(10):2605-17. doi: 10.1007/s11095-014-1358-7. Epub 2014 May 3. Pharm Res. 2014. PMID: 24792824
-
Differences between Physostigmine- and Yohimbine-induced States Are Visualized in Canonical Space Constructed from EEG during Natural Sleep-wake Cycle in Rats.Exp Neurobiol. 2011 Mar;20(1):54-65. doi: 10.5607/en.2011.20.1.54. Epub 2011 Mar 31. Exp Neurobiol. 2011. PMID: 22110362 Free PMC article.
-
Effects of phencyclidine (PCP) and MK 801 on the EEGq in the prefrontal cortex of conscious rats; antagonism by clozapine, and antagonists of AMPA-, alpha(1)- and 5-HT(2A)-receptors.Br J Pharmacol. 2002 Jan;135(1):65-78. doi: 10.1038/sj.bjp.0704451. Br J Pharmacol. 2002. PMID: 11786481 Free PMC article.
References
-
- BALDESSARINI R.J., CENTORRINO F., FLOOD J.G., VOLPICELLI S.A., HUSTON-LYONS D., COHEN B.M. Tissue concentrations of clozapine and its metabolites in the rat. Neuropsychopharmacology. 1993;9:117–124. - PubMed
-
- BEAL S.L., SHEINER L.B. (Eds.) NONMEM users guides. NONMEM project group, University of California, San Francisco, CA; 1992.
-
- BREIMER D.D., DANHOF M. Relevance of the application of pharmacokinetic-pharmacodynamic modelling concepts in drug development. The ‘wooden shoe' paradigm. Clin. Pharmacokinet. 1997;32:259–267. - PubMed
-
- DANHOF M., MANDEMA J.W., HOOGERKAMP A., MATHÔT R.A.A. Pharmacokinetic-pharmacodynamic modelling in pre-clinical investigations: principles and perspectives. Eur. J. Drug Met. Pharm. 1993;18:41–47. - PubMed
-
- DELLA PASQUA O.E., MANDEMA J.W., VOSKUYL R.A., DANHOF M. Pharmacokinetic-pharmacodynamic modelling of the anticonvulsant and electroencephalogram effects of phenytoin in rats. J. Pharmacol. Exp. Ther. 1998;284:460–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

