Formation of nitric oxide from nitroxyl anion: role of quinones and ferricytochrome c
- PMID: 11156574
- PMCID: PMC1572556
- DOI: 10.1038/sj.bjp.0703812
Formation of nitric oxide from nitroxyl anion: role of quinones and ferricytochrome c
Abstract
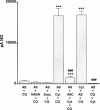
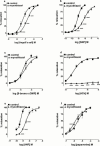
1. Our previous finding that copper ions oxidize nitroxyl anion released from Angeli's salt to nitric oxide prompted us to examine if copper-containing enzymes shared this property. 2. The copper-containing enzyme, tyrosinase, which catalyses the hydroxylation of monophenols to diphenols and the subsequent oxidation of these to the respective unstable quinone, failed to generate nitric oxide from Angeli's salt by itself, but did so in the presence of tyrosine. 3. L-DOPA, the initial product of the reaction of tyrosinase with tyrosine, was not the active species, since it failed to generate nitric oxide from Angeli's salt. Nevertheless, L-DOPA and two other substrates, namely, catechol and tyramine did produce nitric oxide from Angeli's salt in the presence of tyrosinase, suggesting involvement of the respective unstable quinones. In support, we found that 1,4-benzoquinone produced a powerful nitric oxide signal from Angeli's salt. 4. Coenzyme Q(o), an analogue of ubiquinone, failed to generate nitric oxide from Angeli's salt by itself, but produced a powerful signal in the presence of its mitochondrial complex III cofactor, ferricytochrome c. 5. Experiments conducted on rat aortic rings with the mitochondrial complex III inhibitor, myxothiazol, to determine if this pathway was responsible for the vascular conversion of nitroxyl to nitric oxide were equivocal: relaxation to Angeli's salt was inhibited but so too was that to unrelated relaxants. 6. Thus, certain quinones oxidize nitroxyl to nitric oxide. Further work is required to determine if endogenous quinones contribute to the relaxant actions of nitroxyl donors such as Angeli's salt.
Figures




Similar articles
-
Effects of agents that inactivate free radical NO (NO*) on nitroxyl anion-mediated relaxations, and on the detection of NO* released from the nitroxyl anion donor Angeli's salt.Br J Pharmacol. 2001 Oct;134(3):521-8. doi: 10.1038/sj.bjp.0704287. Br J Pharmacol. 2001. PMID: 11588105 Free PMC article.
-
Role of copper ions and cytochrome P450 in the vasodilator actions of the nitroxyl anion generator, Angeli's salt, on rat aorta.Eur J Pharmacol. 2001 Feb 2;412(3):281-9. doi: 10.1016/s0014-2999(00)00845-1. Eur J Pharmacol. 2001. PMID: 11166292
-
Oxidative release of nitric oxide accounts for guanylyl cyclase stimulating, vasodilator and anti-platelet activity of Piloty's acid: a comparison with Angeli's salt.Biochem J. 1995 Dec 1;312 ( Pt 2)(Pt 2):333-9. doi: 10.1042/bj3120333. Biochem J. 1995. PMID: 8526840 Free PMC article.
-
Don't just say no: Differential pathways and pharmacological responses to diverse nitric oxide donors.Biochem Pharmacol. 2018 Oct;156:1-9. doi: 10.1016/j.bcp.2018.08.002. Epub 2018 Aug 3. Biochem Pharmacol. 2018. PMID: 30080991 Review.
-
The chemistry of nitroxyl-releasing compounds.Antioxid Redox Signal. 2011 May 1;14(9):1637-48. doi: 10.1089/ars.2010.3838. Epub 2011 Mar 2. Antioxid Redox Signal. 2011. PMID: 21235345 Free PMC article. Review.
Cited by
-
Acyloxy nitroso compounds as nitroxyl (HNO) donors: kinetics, reactions with thiols, and vasodilation properties.J Med Chem. 2011 Feb 24;54(4):1059-70. doi: 10.1021/jm101432z. Epub 2011 Jan 19. J Med Chem. 2011. PMID: 21247168 Free PMC article.
-
Effects of agents that inactivate free radical NO (NO*) on nitroxyl anion-mediated relaxations, and on the detection of NO* released from the nitroxyl anion donor Angeli's salt.Br J Pharmacol. 2001 Oct;134(3):521-8. doi: 10.1038/sj.bjp.0704287. Br J Pharmacol. 2001. PMID: 11588105 Free PMC article.
-
The pharmacology of nitroxyl (HNO) and its therapeutic potential: not just the Janus face of NO.Pharmacol Ther. 2007 Feb;113(2):442-58. doi: 10.1016/j.pharmthera.2006.11.002. Epub 2006 Nov 29. Pharmacol Ther. 2007. PMID: 17222913 Free PMC article. Review.
-
Biological signaling by small inorganic molecules.Coord Chem Rev. 2016 Jan 1;306(Pt 2):708-723. doi: 10.1016/j.ccr.2015.06.001. Coord Chem Rev. 2016. PMID: 26688591 Free PMC article.
-
Mechanisms of the interaction of nitroxyl with mitochondria.Biochem J. 2004 Apr 15;379(Pt 2):359-66. doi: 10.1042/BJ20031758. Biochem J. 2004. PMID: 14723605 Free PMC article.
References
-
- ARNELLE D.R., STAMLER J.S. NO+, NO, and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 1995;318:279–285. - PubMed
-
- DIERKS E.A., BURSTYN J.N. Nitric oxide (NO.), the only nitrogen monoxide redox form capable of activating soluble guanylate cyclase. Biochem. Pharmacol. 1996;51:1593–1600. - PubMed
-
- DOYLE M.P., MAHAPATRO S.N., BROENE R.D., GUY J.K. Oxidation and reduction of hemoproteins by trioxodinitrate (II). The role of nitrosyl hydride and nitrite. J. Am. Chem. Soc. 1988;110:593–599.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

