Blockade of chloride channels reveals relaxations of rat small mesenteric arteries to raised potassium
- PMID: 11156589
- PMCID: PMC1572528
- DOI: 10.1038/sj.bjp.0703769
Blockade of chloride channels reveals relaxations of rat small mesenteric arteries to raised potassium
Abstract
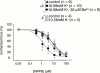
1. Raised extracellular K(+) relaxes some arteries, and has been proposed as Endothelium-Derived Hyperpolarizing Factor (EDHF). However, relaxation of rat small mesenteric arteries to K(+) is highly variable. We have investigated the mechanism of K(+)-induced dilatation and relaxation of pressurized arteries and arteries mounted for measurement of isometric force. 2. Raising [K(+)](o) from 5.88 - 10.58 mM did not dilate or relax pressurized or isometric arteries. Relaxation to raised [K(+)](o) was revealed in the presence of 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB); this effect of NPPB was concentration-dependent (IC(50): 1.16 microM). 3. Relaxations to raised [K(+)](o) in the presence of NPPB, were abolished by 30 microM Ba(2+) or endothelial-denudation. Acetycholine (10 microM) relaxed endothelium-intact arteries in presence of raised [K(+)](o) NPPB and Ba(2+). 4. Relaxations to raised [K(+)](o) were revealed in hyperosmotic superfusate (+60 mM sucrose). These relaxations were abolished by 30 microM Ba(2+). In the presence of raised [K(+)](o), 60 mM sucrose and 30 microM Ba(2+), 10 microM acetycholine still relaxed all arteries. 5. Fifty microM 18 alpha-glycyrrhetinic acid (18 alpha-GA), a gap junction inhibitor, depressed relaxations to both 10 microM acetylcholine and raised [K(+)](o), in the presence of 10 microM NPPB. 6. In summary, blockade of a volume-sensitive Cl(-) conductance in small rat mesenteric arteries, using NPPB or hyperosmotic superfusion, reveals a endothelium-dependent, Ba(2+) sensitive dilatation or relaxation of rat mesenteric arteries to raised [K(+)](o). We conclude that inwardly rectifying potassium channels on the endothelium underlie relaxations to raised [K(+)](o) in rat small mesenteric arteries.
Figures




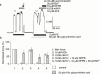

Similar articles
-
Potassium does not mimic EDHF in rat mesenteric arteries.Br J Pharmacol. 2000 Jul;130(5):1174-82. doi: 10.1038/sj.bjp.0703412. Br J Pharmacol. 2000. PMID: 10882404 Free PMC article.
-
Endothelium-dependent vasorelaxation independent of nitric oxide and K(+) release in isolated renal arteries of rats.Br J Pharmacol. 2001 Apr;132(7):1558-64. doi: 10.1038/sj.bjp.0703965. Br J Pharmacol. 2001. PMID: 11264250 Free PMC article.
-
Contribution of K+ channels and ouabain-sensitive mechanisms to the endothelium-dependent relaxations of horse penile small arteries.Br J Pharmacol. 1998 Apr;123(8):1609-20. doi: 10.1038/sj.bjp.0701780. Br J Pharmacol. 1998. PMID: 9605568 Free PMC article.
-
Critical role of gap junctions in endothelium-dependent hyperpolarization in rat mesenteric arteries.Clin Exp Pharmacol Physiol. 2002 Jul;29(7):595-602. doi: 10.1046/j.1440-1681.2002.03689.x. Clin Exp Pharmacol Physiol. 2002. PMID: 12060103
-
Glycyrrhetinic acid-sensitive mechanism does not make a major contribution to non-prostanoid, non-nitric oxide mediated endothelium-dependent relaxation of rat mesenteric artery in response to acetylcholine.Res Commun Mol Pathol Pharmacol. 1999 Mar;103(3):227-39. Res Commun Mol Pathol Pharmacol. 1999. PMID: 10509734
Cited by
-
Boosting the signal: Endothelial inward rectifier K+ channels.Microcirculation. 2017 Apr;24(3):10.1111/micc.12319. doi: 10.1111/micc.12319. Microcirculation. 2017. PMID: 27652592 Free PMC article. Review.
-
Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis?Br J Pharmacol. 2004 Mar;141(6):881-903. doi: 10.1038/sj.bjp.0705698. Br J Pharmacol. 2004. PMID: 15028638 Free PMC article. Review.
-
Inward rectification and vascular function: as it was in the beginning.J Physiol. 2008 Mar 15;586(6):1465-7. doi: 10.1113/jphysiol.2008.151308. Epub 2008 Feb 7. J Physiol. 2008. PMID: 18258660 Free PMC article. Review. No abstract available.
-
K+ potentiates hyperosmolarity-induced vasorelaxations in rat skeletal muscle arterioles.Eur J Appl Physiol. 2006 Apr;96(6):679-85. doi: 10.1007/s00421-005-0128-y. Epub 2006 Jan 17. Eur J Appl Physiol. 2006. PMID: 16416320
-
Suppression of K(+)-induced hyperpolarization by phenylephrine in rat mesenteric artery: relevance to studies of endothelium-derived hyperpolarizing factor.Br J Pharmacol. 2001 Sep;134(1):1-5. doi: 10.1038/sj.bjp.0704256. Br J Pharmacol. 2001. PMID: 11522590 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

