Differential coupling of the human P2Y(11) receptor to phospholipase C and adenylyl cyclase
- PMID: 11156592
- PMCID: PMC1572546
- DOI: 10.1038/sj.bjp.0703788
Differential coupling of the human P2Y(11) receptor to phospholipase C and adenylyl cyclase
Abstract
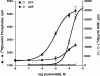
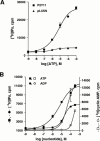
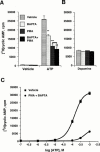
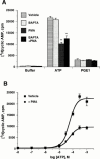
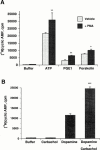
1. The human P2Y(11) (hP2Y(11)) receptor was stably expressed in two cell lines, 1321N1 human astrocytoma cells (1321N1-hP2Y(11)) and Chinese hamster ovary cells (CHO-hP2Y(11)), and its coupling to phospholipase C and adenylyl cyclase was assessed. 2. In 1321N1-hP2Y(11) cells, ATP promoted inositol phosphate (IP) accumulation with low microM potency (EC(50)=8.5+/-0.1 microM), whereas it was 15 fold less potent (130+/-10 microM) in evoking cyclic AMP production. 3. In CHO-hP2Y(11) cells, ATP promoted IP accumulation with slightly higher potency (EC(50)=3.6+/-1.3 microM) than in 1321N1-hP2Y(11) cells, but it was still 15 fold less potent in promoting cyclic AMP accumulation (EC(50)=62.4+/-15.6 microM) than for IP accumulation. Comparable differences in potencies for promoting the two second messenger responses were observed with other adenosine nucleotide analogues. 4. In 1321N1-hP2Y(11) and CHO-hP2Y(11) cells, down regulation of PKC by chronic treatment with phorbol ester decreased ATP-promoted cyclic AMP accumulation by 60--80% (P<0.001) with no change in its potency. Likewise, chelation of intracellular Ca(2+) decreased ATP-promoted cyclic AMP accumulation by approximately 45% in 1321N1-hP2Y(11) cells, whereas chelation had no effect on either the efficacy or potency of ATP in CHO-hP2Y(11) cells. 5. We conclude that coupling of hP2Y(11) receptors to adenylyl cyclase in these cell lines is much weaker than coupling to phospholipase C, and that activation of PKC and intracellular Ca(2+) mobilization as consequences of inositol lipid hydrolysis potentiates the capacity of ATP to increase cyclic AMP accumulation in both 1321N1-hP2Y(11) and CHO-hP2Y(11) cells.
Figures
Similar articles
-
Adenine nucleotide analogues locked in a Northern methanocarba conformation: enhanced stability and potency as P2Y(1) receptor agonists.J Med Chem. 2002 May 9;45(10):2090-100. doi: 10.1021/jm010538v. J Med Chem. 2002. PMID: 11985476 Free PMC article.
-
A molecularly identified P2Y receptor simultaneously activates phospholipase C and inhibits adenylyl cyclase and is nonselectively activated by all nucleoside triphosphates.Mol Pharmacol. 2000 Apr;57(4):805-10. doi: 10.1124/mol.57.4.805. Mol Pharmacol. 2000. PMID: 10727529
-
Pharmacological and second messenger signalling selectivities of cloned P2Y receptors.J Auton Pharmacol. 1996 Dec;16(6):319-23. doi: 10.1111/j.1474-8673.1996.tb00044.x. J Auton Pharmacol. 1996. PMID: 9131407
-
The P2 receptors and congenital platelet function defects.Semin Thromb Hemost. 2005 Apr;31(2):168-73. doi: 10.1055/s-2005-869522. Semin Thromb Hemost. 2005. PMID: 15852220 Review.
-
Evolutionary conservation of the signaling proteins upstream of cyclic AMP-dependent kinase and protein kinase C in gastropod mollusks.Brain Behav Evol. 2009;74(3):191-205. doi: 10.1159/000258666. Epub 2009 Dec 21. Brain Behav Evol. 2009. PMID: 20029183 Free PMC article. Review.
Cited by
-
Pharmacological characterization of the human P2Y11 receptor.Br J Pharmacol. 1999 Nov;128(6):1199-206. doi: 10.1038/sj.bjp.0702909. Br J Pharmacol. 1999. PMID: 10578132 Free PMC article.
-
Ser352 and Ser354 in the carboxyl terminus of the human P2Y(1) receptor are required for agonist-promoted phosphorylation and internalization in MDCK cells.Br J Pharmacol. 2011 Mar;162(6):1304-13. doi: 10.1111/j.1476-5381.2010.01135.x. Br J Pharmacol. 2011. PMID: 21108629 Free PMC article.
-
UTP is not a biased agonist at human P2Y(11) receptors.Purinergic Signal. 2014 Dec;10(4):581-5. doi: 10.1007/s11302-014-9418-3. Purinergic Signal. 2014. PMID: 25015314 Free PMC article.
-
A selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils.Mol Pharmacol. 2013 Jul;84(1):41-9. doi: 10.1124/mol.113.085654. Epub 2013 Apr 16. Mol Pharmacol. 2013. PMID: 23592514 Free PMC article.
-
Invited Lectures : Overviews Purinergic signalling: past, present and future.Purinergic Signal. 2006 May;2(1):1-324. doi: 10.1007/s11302-006-9006-2. Epub 2006 May 15. Purinergic Signal. 2006. PMID: 18404494 Free PMC article. No abstract available.
References
-
- ABOU-SAMRA A.B., JUPPNER H., FORCE T., FREEMAN M.W., KONG X.F., SCHIPANI E., URENA P., RICHARDS J., BONVENTRE J.V., POTTS J.T. , JR, KRONENBERG H.M., SEGRE G.V. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2732–2736. - PMC - PubMed
-
- ALEXANDER S.P.H., CURTIS A.R., HILL S.J., KENDALL D.A. Activation of a metabotropic excitatory amino acid receptor potentiates A2b adenosine receptor-stimulated cyclic AMP accumulation. Neurosci. Lett. 1992;146:231–233. - PubMed
-
- BOYER J.L., DELANEY S.M., VILLANUEVA D., HARDEN T.K. A molecularly identified P2Y receptor simultaneously activates phospholipase C and inhibits adenylyl cyclase and is nonselectively activated by all nucleoside triphosphates. Mol. Pharmacol. 2000;57:805–810. - PubMed
-
- BOYER J.L., WALDO G.L., HARDEN T.K. Molecular cloning and expression of an avian G protein-coupled p2y receptor. Mol. Pharmacol. 1997;52:928–934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

