Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats
- PMID: 11156596
- PMCID: PMC1572554
- DOI: 10.1038/sj.bjp.0703810
Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats
Abstract
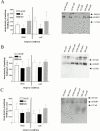
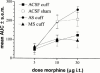
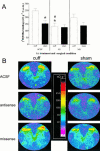
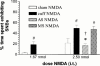
1. Nerve injury often produces long-lasting spontaneous pain, hyperalgesia and allodynia that are refractory to treatment, being only partially relieved by clinical analgesics, and often insensitive to morphine. With the aim of assessing its therapeutic potential, we examined the effect of antisense oligonucleotide knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) in neuropathic rats. 2. We chronically infused rats intrathecally with either vehicle, or 50 microg day(-1) antisense or missense oligonucleotides beginning either 3 days prior to or 5 days after nerve injury. Cold, heat and mechanical sensitivity was assessed prior to any treatment and again every few days after nerve injury. 3. Here we show that knockdown of mGluR(1) significantly reduces cold hyperalgesia, heat hyperalgesia and mechanical allodynia in the ipsilateral (injured) hindpaw of neuropathic rats. 4. Moreover, we show that morphine analgesia is reduced in neuropathic rats, but not in sham-operated rats, and that knockdown of mGluR(1) restores the analgesic efficacy of morphine. 5. We also show that neuropathic rats are more sensitive to the excitatory effects of intrathecally injected N-methyl-D-aspartate (NMDA), and have elevated protein kinase C (PKC) activity in the spinal cord dorsal horn, two effects that are reversed by knockdown of mGluR(1). 6. These results suggest that activity at mGluR(1) contributes to neuropathic pain through interactions with spinal NMDA receptors and PKC, and that knockdown of mGluR(1) may be a useful therapy for neuropathic pain in humans, both to alleviate pain directly, and as an adjunct to opioid analgesic treatment.
Figures


Similar articles
-
Differential effects of NMDA and group I mGluR antagonists on both nociception and spinal cord protein kinase C translocation in the formalin test and a model of neuropathic pain in rats.Pain. 2001 Oct;94(1):17-29. doi: 10.1016/S0304-3959(01)00337-2. Pain. 2001. PMID: 11576741
-
Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve-injured rats.Pain. 1997 May;71(1):57-64. doi: 10.1016/s0304-3959(97)03337-x. Pain. 1997. PMID: 9200174
-
An antisense oligonucleotide to the N-methyl-D-aspartate (NMDA) subunit NMDAR1 attenuates NMDA-induced nociception, hyperalgesia, and morphine tolerance.J Pharmacol Exp Ther. 2005 Feb;312(2):834-40. doi: 10.1124/jpet.104.074856. Epub 2004 Sep 23. J Pharmacol Exp Ther. 2005. PMID: 15388787
-
Spinal cord neuroplasticity following repeated opioid exposure and its relation to pathological pain.Ann N Y Acad Sci. 2001 Mar;933:175-84. doi: 10.1111/j.1749-6632.2001.tb05823.x. Ann N Y Acad Sci. 2001. PMID: 12000019 Review.
-
Implications of intrathecal pertussis toxin animal model on the cellular mechanisms of neuropathic pain syndrome.Acta Anaesthesiol Sin. 2003 Dec;41(4):187-96. Acta Anaesthesiol Sin. 2003. PMID: 14768516 Review.
Cited by
-
Emerging Trends in Pain Modulation by Metabotropic Glutamate Receptors.Front Mol Neurosci. 2019 Jan 4;11:464. doi: 10.3389/fnmol.2018.00464. eCollection 2018. Front Mol Neurosci. 2019. PMID: 30662395 Free PMC article. Review.
-
The sciatic nerve cuffing model of neuropathic pain in mice.J Vis Exp. 2014 Jul 16;(89):51608. doi: 10.3791/51608. J Vis Exp. 2014. PMID: 25078668 Free PMC article.
-
Group I metabotropic glutamate receptors control metaplasticity of spinal cord learning through a protein kinase C-dependent mechanism.J Neurosci. 2008 Nov 12;28(46):11939-49. doi: 10.1523/JNEUROSCI.3098-08.2008. J Neurosci. 2008. PMID: 19005059 Free PMC article.
-
Impaired sensitivity to pain stimuli in plasma membrane calcium ATPase 2 (PMCA2) heterozygous mice: a possible modality- and sex-specific role for PMCA2 in nociception.FASEB J. 2017 Jan;31(1):224-237. doi: 10.1096/fj.201600541R. Epub 2016 Oct 4. FASEB J. 2017. PMID: 27702770 Free PMC article.
-
Effects of mGlu1 receptor blockade on anxiety-related behaviour in the rat lick suppression test.Psychopharmacology (Berl). 2005 Apr;179(1):198-206. doi: 10.1007/s00213-004-2056-7. Epub 2004 Dec 4. Psychopharmacology (Berl). 2005. PMID: 15821950
References
-
- ABE T., SUGIHARA H., NAWA H., SHIGEMOTO R., MIZUNO N., NAKANISHI S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 1992;267:13361–13368. - PubMed
-
- AKHTAR S., AGRAWAL S. In vivo studies with antisense oligonucleotides. Trends Pharmacol. Sci. 1997;18:12–18. - PubMed
-
- AL-GHOUL W.M., LI VOLSI G., WEINBERG R.J., RUSTIONI A. Glutamate immunocytochemistry in the dorsal horn after injury or stimulation of the sciatic nerve of rats. Brain Res. Bull. 1993;30:453–459. - PubMed
-
- ALVAREZ F.I., VILLALBA R.M., CARR P.A., GRANDES P., SOMOHANO P.M. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in rat spinal cord. J. Comp. Neurol. 2000;422:464–487. - PubMed
-
- BERTHELE A., BOXALL S.J., URBAN A., ANNESER J.M., ZIEGLGANSBERGER W., URBAN L., TOLLE T.R. Distribution and developmental changes in metabotropic glutamate receptor messenger RNA expression in the rat lumbar spinal cord. Brain. Res. Dev. Brain. Res. 1999;112:39–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

