Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells
- PMID: 11156605
- PMCID: PMC312602
- DOI: 10.1101/gad.828901
Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells
Abstract
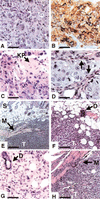
A number of genetic mutations have been identified in human breast cancers, yet the specific combinations of mutations required in concert to form breast carcinoma cells remain unknown. One approach to identifying the genetic and biochemical alterations required for this process involves the transformation of primary human mammary epithelial cells (HMECs) to carcinoma cells through the introduction of specific genes. Here we show that introduction of three genes encoding the SV40 large-T antigen, the telomerase catalytic subunit, and an H-Ras oncoprotein into primary HMECs results in cells that form tumors when transplanted subcutaneously or into the mammary glands of immunocompromised mice. The tumorigenicity of these transformed cells was dependent on the level of ras oncogene expression. Interestingly, transformation of HMECs but not two other human cell types was associated with amplifications of the c-myc oncogene, which occurred during the in vitro growth of the cells. Tumors derived from the transformed HMECs were poorly differentiated carcinomas that infiltrated through adjacent tissue. When these cells were injected subcutaneously, tumors formed in only half of the injections and with an average latency of 7.5 weeks. Mixing the epithelial tumor cells with Matrigel or primary human mammary fibroblasts substantially increased the efficiency of tumor formation and decreased the latency of tumor formation, demonstrating a significant influence of the stromal microenvironment on tumorigenicity. Thus, these observations establish an experimental system for elucidating both the genetic and cell biological requirements for the development of breast cancer.
Figures







References
-
- Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–1260. - PubMed
-
- Bartek J, Durban EM, Hallowes RC, Taylor-Papadimitriou J. A subclass of luminal epithelial cells in the human mammary gland, defined by antibodies to cytokeratins. J Cell Sci. 1985;75:17–33. - PubMed
-
- Bos JL. The ras gene family and human carcinogenesis. Mutat Res. 1988;195:255–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
