The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice
- PMID: 11157056
- PMCID: PMC2195915
- DOI: 10.1084/jem.193.3.365
The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice
Abstract
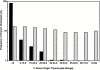
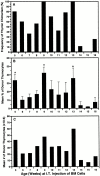
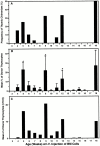
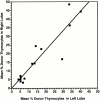
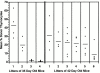
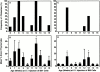
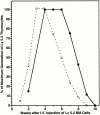
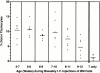
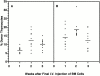
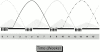
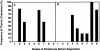
Hematogenous precursors repopulate the thymus of normal adult mice, but it is not known whether this process is continuous or intermittent. Here, two approaches were used to demonstrate that the importation of prothymocytes in adult life is a gated phenomenon. In the first, age-dependent receptivity to thymic chimerism was studied in nonirradiated Ly 5 congenic mice by quantitative intrathymic and intravenous bone marrow (BM) adoptive transfer assays. In the second, the kinetics of importation of blood-borne prothymocytes was determined by timed separation of parabiotic mice. The results showed that >60% of 3-18-wk-old mice developed thymic chimerism after intrathymic injection of BM cells, and that the levels of chimerism (range, 5-90% donor-origin cells) varied cyclically (periodicity, 3 to 5 wk). In contrast, only 11-14% of intravenously injected recipients became chimeric, and chimerism occurred intermittently (receptive period approximately 1 wk; refractory period approximately 3 wk). In the intravenously injected mice, chimerism occurred simultaneously in both thymic lobes; gate opening occurred only after most intrathymic niches for prothymocytes had emptied; and the ensuing wave of thymocytopoiesis encompassed two periods of gating. These kinetics were confirmed in parabiotic mice, and in cohorts of mice in whom gating was synchronized by an initial intrathymic injection of BM cells. In addition, a protocol was developed by which sequential intravenous injections of BM cells over a 3 to 4 wk period routinely induces thymic chimerism in the apparent absence of stem cell chimerism. Hence, the results not only provide a new paradigm for the regulation of prothymocyte importation during adult life, but may also have applied implications for the selective induction of thymocytopoiesis in nonmyeloablated hosts.
Figures
Similar articles
-
Gated importation of prothymocytes by adult mouse thymus is coordinated with their periodic mobilization from bone marrow.J Immunol. 2003 Oct 1;171(7):3568-75. doi: 10.4049/jimmunol.171.7.3568. J Immunol. 2003. PMID: 14500653
-
Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice.J Immunol. 1992 Mar 15;148(6):1604-12. J Immunol. 1992. PMID: 1347301
-
Functional demonstration of intrathymic binding sites and microvascular gates for prothymocytes in irradiated mice.Int Immunol. 2002 Mar;14(3):331-8. doi: 10.1093/intimm/14.3.331. Int Immunol. 2002. PMID: 11867569
-
Two developmentally distinct populations of dendritic cells inhabit the adult mouse thymus: demonstration by differential importation of hematogenous precursors under steady state conditions.J Immunol. 2003 Apr 1;170(7):3514-21. doi: 10.4049/jimmunol.170.7.3514. J Immunol. 2003. PMID: 12646612
-
Cyclical mobilization and gated importation of thymocyte progenitors in the adult mouse: evidence for a thymus-bone marrow feedback loop.Immunol Rev. 2006 Feb;209:58-75. doi: 10.1111/j.0105-2896.2006.00354.x. Immunol Rev. 2006. PMID: 16448534 Review.
Cited by
-
A model 450 million years in the making: zebrafish and vertebrate immunity.Dis Model Mech. 2012 Jan;5(1):38-47. doi: 10.1242/dmm.007138. Dis Model Mech. 2012. PMID: 22228790 Free PMC article. Review.
-
Nonoverlapping functions for Notch1 and Notch3 during murine steady-state thymic lymphopoiesis.Blood. 2011 Sep 1;118(9):2511-9. doi: 10.1182/blood-2011-04-346726. Epub 2011 Jul 18. Blood. 2011. PMID: 21768299 Free PMC article.
-
Paracrine FGF21 dynamically modulates mTOR signaling to regulate thymus function across the lifespan.Nat Aging. 2025 Apr;5(4):588-606. doi: 10.1038/s43587-024-00801-1. Epub 2025 Feb 19. Nat Aging. 2025. PMID: 39972173 Free PMC article.
-
Rapid thymic reconstitution following bone marrow transplantation in neonatal mice is VEGF-dependent.Biol Blood Marrow Transplant. 2012 May;18(5):683-9. doi: 10.1016/j.bbmt.2012.01.006. Epub 2012 Jan 25. Biol Blood Marrow Transplant. 2012. PMID: 22281302 Free PMC article.
-
Developmental dynamics of the neural crest-mesenchymal axis in creating the thymic microenvironment.Sci Adv. 2022 May 13;8(19):eabm9844. doi: 10.1126/sciadv.abm9844. Epub 2022 May 13. Sci Adv. 2022. PMID: 35559672 Free PMC article.
References
-
- Jotereau F.V., LeDouarin N.M. Demonstration of a cyclic renewal of the lymphocyte precursor cells in the quail thymus during embryonic and perinatal life. J. Immunol. 1982;129:1869–1877. - PubMed
-
- Jotereau F.V., Heuze F., Salmon-vie V., Gascan H. Cell kinetics in the fetal mouse thymusprecursor cell input, proliferation, and emigration. J. Immunol. 1987;138:1026–1030. - PubMed
-
- Bechtold T.E., Smith P.B., Turpen J.B. Differential stem cell contributions to thymocyte succession during development of Xenopus laevis . J. Immunol. 1992;148:2975–2982. - PubMed
-
- Shortman K., Wu L. Early T lymphocyte progenitors. Annu. Rev. Immunol. 1996;14:29–47. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

