Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus
- PMID: 11157068
- PMCID: PMC6762333
- DOI: 10.1523/JNEUROSCI.21-03-00823.2001
Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus
Abstract
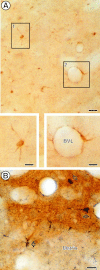
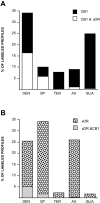
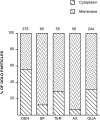
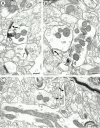
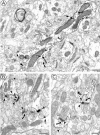
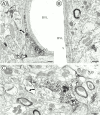
Cannabinoids and opioids are widely consumed drugs of abuse that produce motor depression, in part via respective activation of the cannabinoid subtype 1 receptor (CB1R) and the mu-opioid receptor (muOR), in the striatal circuitry originating in the caudate putamen nucleus (CPN). Thus, the CB1R and muOR may show similar targeting in the CPN. To test this hypothesis, we examined the electron microscopic immunocytochemical labeling of CB1R and muOR in CPN patches of rat brain. Of the CB1R-labeled profiles, 34% (588) were dendrites, presumably arising from spiny as well as aspiny-type somata, which also contained CB1R immunoreactivity. In dendrites, CB1R often was localized to nonsynaptic and synaptic plasma membranes, particularly near asymmetric excitatory-type junctions. Almost one-half of the CB1R-labeled dendrites contained muOR immunoreactivity, whereas only 20% of all muOR-labeled dendrites expressed CB1R. Axons and axon terminals as well as abundant glial processes also showed plasmalemmal CB1R and were mainly without muOR immunoreactivity. Many CB1R-labeled axon terminals were small and without recognizable synaptic junctions, but a few also formed asymmetric, or more rarely symmetric, synapses. The CB1R-labeled glial processes were often perivascular or perisynaptic, surrounding asymmetric excitatory-type axospinous synapses. Our results show that in CPN patches CB1R and muOR are targeted strategically to some of the same postsynaptic neurons, which may account for certain similarities in motor function. Furthermore, they also provide evidence that CB1R may play a major role in the modulation of presynaptic transmitter release and glial functions that are unaffected in large part by opioids active at muOR in CPN.
Figures


Similar articles
-
Mu-opioid and NMDA-type glutamate receptors are often colocalized in spiny neurons within patches of the caudate-putamen nucleus.J Comp Neurol. 1999 Sep 13;412(1):132-46. doi: 10.1002/(sici)1096-9861(19990913)412:1<132::aid-cne10>3.0.co;2-b. J Comp Neurol. 1999. PMID: 10440715
-
Ultrastructural immunocytochemical localization of mu-opioid receptors in dendritic targets of dopaminergic terminals in the rat caudate-putamen nucleus.Neuroscience. 1997 Dec;81(3):757-71. doi: 10.1016/s0306-4522(97)00253-4. Neuroscience. 1997. PMID: 9316027
-
Postnatal development of mu-opioid receptors in the rat caudate-putamen nucleus parallels asymmetric synapse formation.Neuroscience. 2003;118(3):695-708. doi: 10.1016/s0306-4522(02)00926-0. Neuroscience. 2003. PMID: 12710977
-
Ultrastructural immunocytochemical localization of mu opioid receptors and Leu5-enkephalin in the patch compartment of the rat caudate-putamen nucleus.J Comp Neurol. 1996 Nov 25;375(4):659-74. doi: 10.1002/(SICI)1096-9861(19961125)375:4<659::AID-CNE7>3.0.CO;2-0. J Comp Neurol. 1996. PMID: 8930791
-
Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter.J Comp Neurol. 1999 Jul 26;410(2):197-210. doi: 10.1002/(sici)1096-9861(19990726)410:2<197::aid-cne3>3.0.co;2-d. J Comp Neurol. 1999. PMID: 10414527 Review.
Cited by
-
Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans.J Addict Med. 2015 May-Jun;9(3):204-10. doi: 10.1097/ADM.0000000000000118. J Addict Med. 2015. PMID: 25748562 Free PMC article. Clinical Trial.
-
Frequent Cannabis Use and Cessation of Injection of Opioids, Vancouver, Canada, 2005-2018.Am J Public Health. 2020 Oct;110(10):1553-1560. doi: 10.2105/AJPH.2020.305825. Epub 2020 Aug 20. Am J Public Health. 2020. PMID: 32816538 Free PMC article.
-
Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling.Proc Natl Acad Sci U S A. 2006 May 23;103(21):8251-6. doi: 10.1073/pnas.0510797103. Epub 2006 May 12. Proc Natl Acad Sci U S A. 2006. PMID: 16698932 Free PMC article.
-
Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis.J Neurosci. 2005 Mar 16;25(11):2874-84. doi: 10.1523/JNEUROSCI.4232-04.2005. J Neurosci. 2005. PMID: 15772347 Free PMC article.
-
Cannabinoid receptors and endocannabinoids: evidence for new players.AAPS J. 2006 Apr 28;8(2):E298-306. doi: 10.1007/BF02854900. AAPS J. 2006. PMID: 16796380 Free PMC article. Review.
References
-
- Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–1072. - PubMed
-
- Auclair N, Otani S, Soubrié P, Crépel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. - PubMed
-
- Bouaboula M, Bourrié B, Rinaldi-Carmona M, Shire D, Le Fur G, Casellas P. Stimulation of cannabinoid receptor CB1 induces krox-24 expression in human astrocytoma cells. J Biol Chem. 2000;270:13973–13980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
