Co-expression of putative pheromone receptors in the sensory neurons of the vomeronasal organ
- PMID: 11157070
- PMCID: PMC6762303
- DOI: 10.1523/JNEUROSCI.21-03-00843.2001
Co-expression of putative pheromone receptors in the sensory neurons of the vomeronasal organ
Abstract
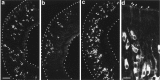
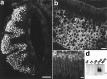
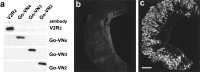
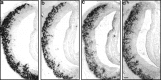
Two large and divergent families of G-protein-coupled receptors (V1Rs and V2Rs) are expressed in subsets of neurons in the vomeronasal organ. These receptors are likely to mediate pheromone responses, but it appears that many V2R genes may encode expressed pseudogenes rather than functional proteins. Therefore we have raised antibodies to representative V2Rs and show labeling of vomeronasal neurons demonstrating that V2R genes encode expressed receptors. V2R immunoreactivity was detected at the sensory surface of the vomeronasal organ in dendritic terminals, indicating that these receptors are capable of directly interacting with pheromones and mediating physiological responses. Immunohistochemistry confirmed that three V2R receptors are expressed in small subsets of sensory neurons. However, surprisingly we found that a subfamily of V2R genes is broadly expressed in the Goalpha-layer of the vomeronasal organ and are coexpressed in the same cells as other V2Rs. This is in direct contrast to the main olfactory epithelium where sensory neurons express only a single receptor. Thus, our results suggest that different modes of the information processing may occur in the main and accessory olfactory systems.
Figures
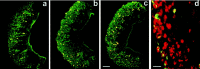
Similar articles
-
Expression of pheromone receptor gene families during olfactory development in the mouse: expression of a V1 receptor in the main olfactory epithelium.Eur J Neurosci. 2006 May;23(10):2563-72. doi: 10.1111/j.1460-9568.2006.04795.x. Eur J Neurosci. 2006. PMID: 16817859
-
Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules.Cell. 2003 Mar 7;112(5):607-18. doi: 10.1016/s0092-8674(03)00153-3. Cell. 2003. PMID: 12628182
-
A novel family of candidate pheromone receptors in mammals.Neuron. 2000 Dec;28(3):835-45. doi: 10.1016/s0896-6273(00)00157-4. Neuron. 2000. PMID: 11163270
-
Pheromone detection in rodents.Neuroreport. 2001 Oct 8;12(14):A81-4. doi: 10.1097/00001756-200110080-00001. Neuroreport. 2001. PMID: 11568658 Review. No abstract available.
-
Expression of candidate pheromone receptor genes in vomeronasal neurons.Chem Senses. 1998 Aug;23(4):467-75. doi: 10.1093/chemse/23.4.467. Chem Senses. 1998. PMID: 9759535 Review.
Cited by
-
Distribution of cells expressing vomeronasal receptors in the olfactory organ of turtles.J Vet Med Sci. 2020 Aug 19;82(8):1068-1079. doi: 10.1292/jvms.20-0207. Epub 2020 Jul 28. J Vet Med Sci. 2020. PMID: 32727968 Free PMC article.
-
Ancestral amphibian v2rs are expressed in the main olfactory epithelium.Proc Natl Acad Sci U S A. 2013 May 7;110(19):7714-9. doi: 10.1073/pnas.1302088110. Epub 2013 Apr 23. Proc Natl Acad Sci U S A. 2013. PMID: 23613591 Free PMC article.
-
Ancient and Nonuniform Loss of Olfactory Receptor Expression Renders the Shark Nose a De Facto Vomeronasal Organ.Mol Biol Evol. 2023 Apr 4;40(4):msad076. doi: 10.1093/molbev/msad076. Mol Biol Evol. 2023. PMID: 36971115 Free PMC article.
-
Expression of nonclassical class I major histocompatibility genes defines a tripartite organization of the mouse vomeronasal system.J Neurosci. 2008 Mar 5;28(10):2332-41. doi: 10.1523/JNEUROSCI.4807-07.2008. J Neurosci. 2008. PMID: 18322080 Free PMC article.
-
In-depth Physiological Analysis of Defined Cell Populations in Acute Tissue Slices of the Mouse Vomeronasal Organ.J Vis Exp. 2016 Sep 10;(115):54517. doi: 10.3791/54517. J Vis Exp. 2016. PMID: 27684435 Free PMC article.
References
-
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. - PubMed
-
- Belluscio L, Koentges G, Axel R, Dulac C. A map of pheromone receptor activation in the mammalian brain. Cell. 1999;97:209–220. - PubMed
-
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
