The information content of spontaneous retinal waves
- PMID: 11157082
- PMCID: PMC6762322
- DOI: 10.1523/JNEUROSCI.21-03-00961.2001
The information content of spontaneous retinal waves
Abstract
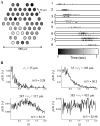
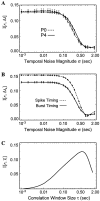
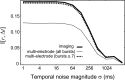
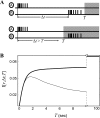
Spontaneous neural activity that is present in the mammalian retina before the onset of vision is required for the refinement of retinotopy in the lateral geniculate nucleus and superior colliculus. This paper explores the information content of this retinal activity, with the goal of determining constraints on the nature of the developmental mechanisms that use it. Through information-theoretic analysis of multielectrode and calcium-imaging experiments, we show that the spontaneous retinal activity present early in development provides information about the relative positions of retinal ganglion cells and can, in principle, be used at retinogeniculate and retinocollicular synapses to refine retinotopy. Remarkably, we find that most retinotopic information provided by retinal waves exists on relatively coarse time scales, suggesting that developmental mechanisms must be sensitive to timing differences from 100 msec up to 2 sec to make optimal use of it. In fact, a simple Hebbian-type learning rule with a correlation window on the order of seconds is able to extract the bulk of the available information. These findings are consistent with bursts of action potentials (rather than single spikes) being the unit of information used during development and suggest new experimental approaches for studying developmental plasticity of the retinogeniculate and retinocollicular synapses. More generally, these results demonstrate how the properties of neuronal systems can be inferred from the statistics of their input.
Figures


References
-
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. - PubMed
-
- Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6:119–126. - PubMed
-
- Borst A, Theunissen FE. Information theory and neural coding. Nat Neurosci. 1999;2:947–957. - PubMed
-
- Cook JE, Becker DL. Spontaneous activity as a determinant of axonal connections. Eur J Neurosci. 1990;2:162–169. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
