Brief treatments with forskolin enhance s-phase entry in balance epithelia from the ears of rats
- PMID: 11157083
- PMCID: PMC6762301
- DOI: 10.1523/JNEUROSCI.21-03-00974.2001
Brief treatments with forskolin enhance s-phase entry in balance epithelia from the ears of rats
Abstract
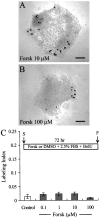
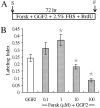
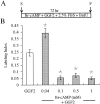
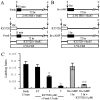
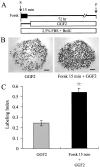
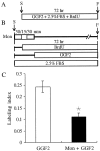
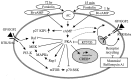
In the ears of mammals, hair cell loss results in permanent hearing and balance deficits, whereas in fish, amphibians, and birds, the production of replacement hair cells can restore those modalities. In avian ears, continuous exposures to forskolin trigger cell proliferation and the regeneration of hair cells, so we investigated the effect of forskolin on sensory epithelia cultured from the ears of mammals. Continuous 72 hr exposures to forskolin failed to induce proliferation in neonatal rat utricles, but brief (</=1 hr) exposures to forskolin or Br-cAMP did. Proliferation occurred only in media that contained serum. Forskolin also augmented the mitogenic effects of glial growth factor 2. The S-phase entry induced by forskolin was blocked by monensin and bafilomycin, two compounds that can inhibit the recycling of membrane receptors. The results are consistent with the hypothesis that in mammalian vestibular epithelia elevated cAMP induces S-phase entry by increasing the number of growth factor receptors at the plasma membrane.
Figures



Similar articles
-
Intracellular signals that control cell proliferation in mammalian balance epithelia: key roles for phosphatidylinositol-3 kinase, mammalian target of rapamycin, and S6 kinases in preference to calcium, protein kinase C, and mitogen-activated protein kinase.J Neurosci. 2001 Jan 15;21(2):570-80. doi: 10.1523/JNEUROSCI.21-02-00570.2001. J Neurosci. 2001. PMID: 11160436 Free PMC article.
-
Induction of cell proliferation and beta-catenin expression in rat utricles in vitro.Acta Otolaryngol Suppl. 2004 Mar;(551):22-5. doi: 10.1080/03655230310016672. Acta Otolaryngol Suppl. 2004. PMID: 15078072
-
Heregulin enhances regenerative proliferation in postnatal rat utricular sensory epithelium after ototoxic damage.J Neurocytol. 1999 Oct-Nov;28(10-11):901-12. doi: 10.1023/a:1007078307638. J Neurocytol. 1999. PMID: 10900093
-
Transforming growth factor-beta1 and glial growth factor 2 reduce neurotrophin-3 mRNA expression in cultured Schwann cells via a cAMP-dependent pathway.Brain Res Mol Brain Res. 1999 Aug 25;71(2):256-64. doi: 10.1016/s0169-328x(99)00200-4. Brain Res Mol Brain Res. 1999. PMID: 10521580
-
Ongoing cell death and immune influences on regeneration in the vestibular sensory organs.Ann N Y Acad Sci. 2001 Oct;942:34-45. doi: 10.1111/j.1749-6632.2001.tb03733.x. Ann N Y Acad Sci. 2001. PMID: 11710476 Review.
Cited by
-
A historical to present-day account of efforts to answer the question: "what puts the brakes on mammalian hair cell regeneration?".Hear Res. 2013 Mar;297:52-67. doi: 10.1016/j.heares.2013.01.005. Epub 2013 Jan 17. Hear Res. 2013. PMID: 23333259 Free PMC article. Review.
-
Cell density and N-cadherin interactions regulate cell proliferation in the sensory epithelia of the inner ear.J Neurosci. 2002 Apr 1;22(7):2607-16. doi: 10.1523/JNEUROSCI.22-07-02607.2002. J Neurosci. 2002. PMID: 11923426 Free PMC article.
-
Has hair cell loss MET its match?Proc Natl Acad Sci U S A. 2007 Oct 16;104(42):16400-1. doi: 10.1073/pnas.0708154104. Epub 2007 Oct 9. Proc Natl Acad Sci U S A. 2007. PMID: 17925436 Free PMC article. No abstract available.
-
Inner ear hair cells produced in vitro by a mesenchymal-to-epithelial transition.Proc Natl Acad Sci U S A. 2007 Oct 16;104(42):16675-80. doi: 10.1073/pnas.0704576104. Epub 2007 Sep 25. Proc Natl Acad Sci U S A. 2007. PMID: 17895386 Free PMC article.
-
Reprogramming by drug-like molecules leads to regeneration of cochlear hair cell-like cells in adult mice.Proc Natl Acad Sci U S A. 2023 Apr 25;120(17):e2215253120. doi: 10.1073/pnas.2215253120. Epub 2023 Apr 17. Proc Natl Acad Sci U S A. 2023. PMID: 37068229 Free PMC article.
References
-
- Basu SK, Goldstein JL, Anderson RG, Brown MS. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981;24:493–502. - PubMed
-
- Burgering BM, Bos JL. Regulation of Ras-mediated signalling: more than one way to skin a cat. Trends Biochem Sci. 1995;20:18–22. - PubMed
-
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. - PubMed
-
- Corwin JT, Oberholtzer JC. Fish n' chicks: model recipes for hair-cell regeneration? Neuron. 1997;19:951–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
