Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease
- PMID: 11157088
- PMCID: PMC6762327
- DOI: 10.1523/JNEUROSCI.21-03-01033.2001
Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease
Abstract
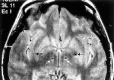
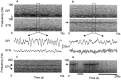
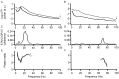
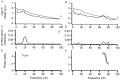
The extent of synchronization within and between the nuclei of the basal ganglia is unknown in Parkinson's disease. The question is an important one because synchronization will increase postsynaptic efficacy at subsequent projection targets. We simultaneously recorded local potentials (LPs) from the globus pallidus interna (GPi) and subthalamic nucleus (STN) in four awake patients after neurosurgery for Parkinson's disease. Nuclei from both sides were recorded in two patients so that a total of six ipsilateral GPi-STN LP recordings were made. Without medication, the power within and the coherence between the GPi and STN was dominated by activity with a frequency <30 Hz. Treatment with the dopamine precursor levodopa reduced the low-frequency activity and resulted in a new peak at approximately 70 Hz. This was evident in the power spectrum from STN and GPi and in the coherence between these nuclei. The phase relationship between the nuclei varied in a complex manner according to frequency band and the presence of exogenous dopaminergic stimulation. Synchronization of activity does occur between pallidum and STN, and its pattern is critically dependent on the level of dopaminergic activity.
Figures


References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–376. - PubMed
-
- Alexander GE, Crutcher ME. Functional architecture of the basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods. 1997;73:69–79. - PubMed
-
- Bejjani B, Damier P, Arnulf I, Bonnet AM, Vidailhet M, Dormont D, Pidoux B, Cornu P, Marsault C, Agid Y. Pallidal stimulation for Parkinson's disease: two targets? Neurology. 1997;49:1564–1569. - PubMed
-
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
