Elevation of nerve growth factor and antisense knockdown of TrkA receptor during contextual memory consolidation
- PMID: 11157090
- PMCID: PMC6762334
- DOI: 10.1523/JNEUROSCI.21-03-01047.2001
Elevation of nerve growth factor and antisense knockdown of TrkA receptor during contextual memory consolidation
Abstract
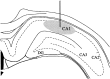
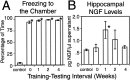
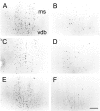
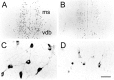
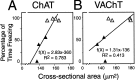
We report here a series of experiments establishing a role for nerve growth factor and its high-affinity receptor TrkA in contextual memory consolidation. In all experiments, we trained rats in a novel chamber using tone and shock. Our first experiment revealed that endogenous nerve growth factor (NGF) increases in the hippocampus at a critical time during consolidation that occurs 1 week after training. NGF levels at other intervals (24 hr and 2 and 4 weeks after training) did not differ from those of naive control animals. In our second experiment, we blocked effects that NGF has at 1 week after training by infusing antisense TrkA phosphorothioate DNA oligonucleotide. Reduction of septohippocampal TrkA receptor expression selectively impaired memory consolidation for context but not for tone. Animals with antisense TrkA oligonucleotide infused into the medial septal area or CA1 of the hippocampus froze less when placed in the training chamber than did animals infused with inactive randomized oligonucleotide. At 4 weeks after training, antisense TrkA oligonucleotide had no effect on freezing. Third, we correlated levels of freezing with choline acetyltransferase (ChAT) and vesicular acetylcholine transporter (VAChT) immunohistochemistry. Antisense TrkA infused into CA1 of the hippocampus reduced cell body cross-sectional area for cholinergic cells in the medial septal area and decreased the density of hippocampal terminals labeled for ChAT and VAChT proteins. Cholinergic cell body measurements were significantly correlated with freezing. Taken together, these results indicate a role for nerve growth factor acting via the TrkA receptor on ChAT and VAChT proteins in contextual memory consolidation.
Figures


Similar articles
-
Activation of TrkA by nerve growth factor upregulates expression of the cholinergic gene locus but attenuates the response to ciliary neurotrophic growth factor.Biochem J. 1999 Sep 1;342 ( Pt 2)(Pt 2):301-8. Biochem J. 1999. PMID: 10455015 Free PMC article.
-
A nerve growth factor mimetic TrkA antagonist causes withdrawal of cortical cholinergic boutons in the adult rat.Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):4067-72. doi: 10.1073/pnas.96.7.4067. Proc Natl Acad Sci U S A. 1999. PMID: 10097164 Free PMC article.
-
Coordinate expression of the vesicular acetylcholine transporter and choline acetyltransferase following septohippocampal pathway lesions.J Neurochem. 1998 Dec;71(6):2411-20. doi: 10.1046/j.1471-4159.1998.71062411.x. J Neurochem. 1998. PMID: 9832139
-
Repeated nicotine exposure in rats: effects on memory function, cholinergic markers and nerve growth factor.Neuroscience. 2005;130(4):997-1012. doi: 10.1016/j.neuroscience.2004.10.006. Neuroscience. 2005. PMID: 15652996
-
Dexamethasone induces TrkA and p75NTR immunoreactivity in the cerebral cortex and hippocampus.Exp Neurol. 2000 Apr;162(2):257-67. doi: 10.1006/exnr.2000.7360. Exp Neurol. 2000. PMID: 10739632
Cited by
-
Thyroid receptor β involvement in the effects of acute nicotine on hippocampus-dependent memory.Neuropharmacology. 2015 Jun;93:155-63. doi: 10.1016/j.neuropharm.2015.01.026. Epub 2015 Feb 7. Neuropharmacology. 2015. PMID: 25666034 Free PMC article.
-
New perspectives on the basal forebrain cholinergic system in Alzheimer's disease.Neurosci Biobehav Rev. 2023 Jul;150:105192. doi: 10.1016/j.neubiorev.2023.105192. Epub 2023 Apr 20. Neurosci Biobehav Rev. 2023. PMID: 37086935 Free PMC article. Review.
-
Dietary Polyphenol Supplementation Prevents Alterations of Spatial Navigation in Middle-Aged Mice.Front Behav Neurosci. 2016 Feb 9;10:9. doi: 10.3389/fnbeh.2016.00009. eCollection 2016. Front Behav Neurosci. 2016. PMID: 26903826 Free PMC article.
-
NGF is essential for hippocampal plasticity and learning.J Neurosci. 2009 Sep 2;29(35):10883-9. doi: 10.1523/JNEUROSCI.2594-09.2009. J Neurosci. 2009. PMID: 19726646 Free PMC article.
-
Evaluation of systemic administration of Boswellia papyrifera extracts on spatial memory retention in male rats.J Nat Med. 2011 Jul;65(3-4):519-25. doi: 10.1007/s11418-011-0533-y. Epub 2011 Apr 11. J Nat Med. 2011. PMID: 21479965
References
-
- Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378:454–467. - PubMed
-
- Bejanin S, Cervini R, Mallet J, Berrard S. A unique gene organization for two cholinergic markers, choline acetyltransferase and a putative vesicular transporter of acetylcholine. J Biol Chem. 1994;269:21944–21947. - PubMed
-
- Berrard S, Varoqui H, Cervini R, Israël M, Mallet J, Diebler MF. Coregulation of two embedded gene products choline acetyltransferase and the vesicular acetylcholine transporter. J Neurochem. 1995;65:939–942. - PubMed
-
- Berse B, Blusztajn JK. Coordinated up-regulation of choline acetyltransferase and vesicular acetylcholine transporter gene expression by the retinoic acid receptor alpha cAMP and leukemia inhibitory factor/ciliary neurotrophic factor signaling pathways in a murine septal cell line. J Biol Chem. 1995;270:22101–22104. - PubMed
-
- Butcher LL, Oh JD, Woolf NJ, Edwards RH, Roghani A. Organization of central cholinergic neurons revealed by combined in situ hybridization histochemistry and choline-O-acetyltransferase immunocytochemistry. Neurochem Int. 1992;21:429–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
