Impact of dilution on microbial community structure and functional potential: comparison of numerical simulations and batch culture experiments
- PMID: 11157234
- PMCID: PMC92638
- DOI: 10.1128/AEM.67.2.702-712.2001
Impact of dilution on microbial community structure and functional potential: comparison of numerical simulations and batch culture experiments
Abstract
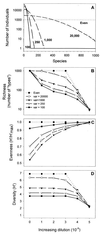
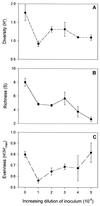
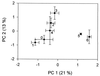
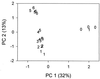
A series of microcosm experiments was performed using serial dilutions of a sewage microbial community to inoculate a set of batch cultures in sterile sewage. After inoculation, the dilution-defined communities were allowed to regrow for several days and a number of community attributes were measured in the regrown assemblages. Based upon a set of numerical simulations, community structure was expected to differ along the dilution gradient; the greatest differences in structure were anticipated between the undiluted-low-dilution communities and the communities regrown from the very dilute (more than 10(-4)) inocula. Furthermore, some differences were expected among the lower-dilution treatments (e.g., between undiluted and 10(-1)) depending upon the evenness of the original community. In general, each of the procedures used to examine the experimental community structures separated the communities into at least two, often three, distinct groups. The groupings were consistent with the simulated dilution of a mixture of organisms with a very uneven distribution. Significant differences in community structure were detected with genetic (amplified fragment length polymorphism and terminal restriction fragment length polymorphism), physiological (community level physiological profiling), and culture-based (colony morphology on R2A agar) measurements. Along with differences in community structure, differences in community size (acridine orange direct counting), composition (ratio of sewage medium counts to R2A counts, monitoring of each colony morphology across the treatments), and metabolic redundancy (i.e., generalist versus specialist) were also observed, suggesting that the differences in structure and diversity of communities maintained in the same environment can be manifested as differences in community organization and function.
Figures

References
-
- Bej A K, Mahbubani M H. Applications of the polymerase chain reaction (PCR) in vitro DNA-amplification method in environmental microbiology. In: Griffin H G, Griffin A M, editors. PCR technology: current innovations. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 327–339.
-
- Busse H-J, Denner E B M, Lubitz W. Classification and identification of bacteria: current approaches to an old problem. Overview of methods used in bacterial systematics. J Biotechnol. 1996;47:3–38. - PubMed
-
- Chapin F S, Walker B H, Hobbs R J, Hooper D U, Lawton J H, Sala O E, Tilman D. Biotic control over the functioning of ecosystems. Science. 1997;277:500–503.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

