Nisin resistance of Streptococcus bovis
- PMID: 11157247
- PMCID: PMC92651
- DOI: 10.1128/AEM.67.2.808-813.2001
Nisin resistance of Streptococcus bovis
Abstract
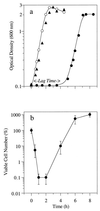
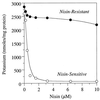
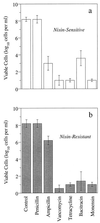
The growth of Streptococcus bovis JB1 was initially inhibited by nisin (1 microM), and nisin caused a more than 3-log decrease in viability. However, some of the cells survived, and these nisin-resistant cells grew as rapidly as untreated ones. To see if the nisin resistance was merely a selection, nisin-sensitive cells were obtained from agar plates lacking nisin. Results indicated that virtually any nisin-sensitive cell could become nisin-resistant if the ratio of nisin to cells was not too high and the incubation period was long enough. Isolates obtained from the rumen were initially nisin sensitive, but they also developed nisin resistance. Nisin-resistant cultures remained nisin resistant even if nisin was not present, but competition studies indicated that nisin-sensitive cells could eventually displace the resistant ones if nisin was not present. Nisin-sensitive, glucose-energized cells lost virtually all of their intracellular potassium if 1 microM nisin was added, but resistant cells retained potassium even after addition of 10 microM nisin. Nisin-resistant cells were less hydrophobic and more lysozyme-resistant than nisin-sensitive cells. Because the nisin-resistant cells bound less cytochrome c, it appeared that nisin was being excluded by a net positive (i.e., less negative) charge. Nisin-resistant cells had more lipoteichoic acid than nisin-sensitive cells, and deesterified lipoteichoic acids from nisin-resistant cells migrated more slowly through a polyacrylamide gel than those from nisin-sensitive cells. These results indicated that lipoteichoic acids could be modified to increase the resistance of S. bovis to nisin. S. bovis JB1 cultures were still sensitive to monensin, tetracycline, vancomycin, and bacitracin, but ampicillin resistance was 1,000-fold greater.
Figures





Similar articles
-
The ability of non-bacteriocin producing Streptococcus bovis strains to bind and transfer bovicin HC5 to other sensitive bacteria.Anaerobe. 2009 Aug;15(4):168-72. doi: 10.1016/j.anaerobe.2008.10.002. Epub 2009 Jan 3. Anaerobe. 2009. PMID: 19171197
-
High prevalence of inducible erythromycin resistance among Streptococcus bovis isolates in Taiwan.Antimicrob Agents Chemother. 2001 Dec;45(12):3362-5. doi: 10.1128/AAC.45.12.3362-3365.2001. Antimicrob Agents Chemother. 2001. PMID: 11709309 Free PMC article.
-
The activity and stability of cell-associated activity of bovicin HC5, a bacteriocin from Streptococcus bovis HC5.FEMS Microbiol Lett. 2008 Jun;283(2):162-6. doi: 10.1111/j.1574-6968.2008.01155.x. Epub 2008 Apr 24. FEMS Microbiol Lett. 2008. PMID: 18445020
-
The effect of calcium and magnesium on the activity of bovicin HC5 and nisin.Curr Microbiol. 2006 Nov;53(5):365-9. doi: 10.1007/s00284-005-0417-z. Epub 2006 Oct 11. Curr Microbiol. 2006. PMID: 17036211
-
The bacteriocins of ruminal bacteria and their potential as an alternative to antibiotics.J Mol Microbiol Biotechnol. 2002 Jul;4(4):347-55. J Mol Microbiol Biotechnol. 2002. PMID: 12125815 Review.
Cited by
-
Bovicins: The Bacteriocins of Streptococci and Their Potential in Methane Mitigation.Probiotics Antimicrob Proteins. 2019 Dec;11(4):1403-1413. doi: 10.1007/s12602-018-9502-z. Probiotics Antimicrob Proteins. 2019. PMID: 30603877 Review.
-
Resistance of rumen bacteria murein to bovine gastric lysozyme.BMC Ecol. 2004 May 11;4:7. doi: 10.1186/1472-6785-4-7. BMC Ecol. 2004. PMID: 15137912 Free PMC article.
-
Plasmid Complement of Lactococcus lactis NCDO712 Reveals a Novel Pilus Gene Cluster.PLoS One. 2016 Dec 12;11(12):e0167970. doi: 10.1371/journal.pone.0167970. eCollection 2016. PLoS One. 2016. PMID: 27941999 Free PMC article.
-
Transcriptome analysis reveals mechanisms by which Lactococcus lactis acquires nisin resistance.Antimicrob Agents Chemother. 2006 May;50(5):1753-61. doi: 10.1128/AAC.50.5.1753-1761.2006. Antimicrob Agents Chemother. 2006. PMID: 16641446 Free PMC article.
-
Novel mechanism for nisin resistance via proteolytic degradation of nisin by the nisin resistance protein NSR.Antimicrob Agents Chemother. 2009 May;53(5):1964-73. doi: 10.1128/AAC.01382-08. Epub 2009 Mar 9. Antimicrob Agents Chemother. 2009. PMID: 19273681 Free PMC article.
References
-
- Breuer B, Radler F. Inducible resistance against nisin in Lactobacillus casei. Arch Microbiol. 1996;165:114–118.
-
- Breukink E, Wiedemann I, van Kraaij C, Kuipers O P, Sahl H-G, de Kruijff B. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science. 1999;286:2361–2364. - PubMed
-
- Callaway T R, Carneiro De Melo A M S, Russell J B. The effect of nisin and monensin on ruminal fermentations in vitro. Curr Microbiol. 1997;35:90–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

