CD13/APN is activated by angiogenic signals and is essential for capillary tube formation
- PMID: 11157481
- PMCID: PMC4470622
- DOI: 10.1182/blood.v97.3.652
CD13/APN is activated by angiogenic signals and is essential for capillary tube formation
Abstract
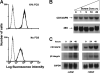
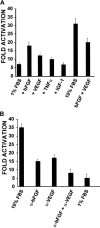
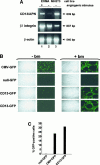
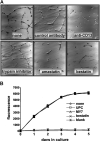
In the hematopoietic compartment, the CD13/APN metalloprotease is one of the earliest markers of cells committed to the myeloid lineage where it is expressed exclusively on the surface of myeloid progenitors and their differentiated progeny. CD13/APN is also found in nonhematopoietic tissues, and its novel expression on the endothelial cells of angiogenic, but not normal, vasculature was recently described. Treatment of animals with CD13/APN inhibitors significantly impaired retinal neovascularization, chorioallantoic membrane angiogenesis, and xenograft tumor growth, indicating that CD13/APN plays an important functional role in vasculogenesis and identifying it as a critical regulator of angiogenesis. To investigate the mechanisms of CD13/APN induction in tumor vasculature, the regulation of CD13/APN by factors contributing to angiogenic progression was studied. In this report, it is shown that endogenous CD13/APN levels in primary cells and cell lines are up-regulated in response to hypoxia, angiogenic growth factors, and signals regulating capillary tube formation during angiogenesis. Transcription of reporter plasmids containing CD13/APN proximal promoter sequences is significantly increased in response to the same angiogenic signals that regulate the expression of the endogenous gene and in human tumor xenografts, indicating that this fragment contains elements essential for the angiogenic induction of CD13/APN expression. Finally, functional antagonists of CD13/APN interfere with tube formation but not proliferation of primary vascular endothelial cells, suggesting that CD13/APN functions in the control of endothelial cell morphogenesis. These studies clearly establish the CD13/APN metalloprotease as an important regulator of endothelial morphogenesis during angiogenesis.
Figures
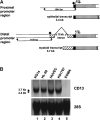
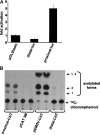
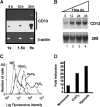
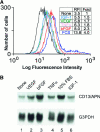
References
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. - PubMed
-
- Hogg N, Horton MJ. Myeloid antigens: new and previously defined clusters. In: McMichael AJ, editor. Leukocyte Typing III: Proceedings of the Third International Workshop on Human Leukocyte Differentiation Antigens. Oxford University Press; New York, NY: 1987. pp. 576–621.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

