The human cytomegalovirus gene product US6 inhibits ATP binding by TAP
- PMID: 11157746
- PMCID: PMC133477
- DOI: 10.1093/emboj/20.3.387
The human cytomegalovirus gene product US6 inhibits ATP binding by TAP
Abstract
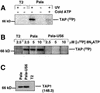
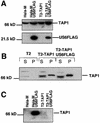
Human cytomegalovirus (HCMV) encodes several genes that disrupt the major histocompatibility complex (MHC) class I antigen presentation pathway. We recently described the HCMV-encoded US6 gene product, a 23 kDa endoplasmic reticulum (ER)-resident type I integral membrane protein that binds to the transporter associated with antigen processing (TAP), inhibits peptide translocation and prevents MHC class I assembly. The functional consequence of this inhibition is to prevent the cell surface expression of class I bound viral peptides and their recognition by HCMV-specific cytotoxic T cells. Here we describe a novel mechanism of action for US6. We demonstrate that US6 inhibits the binding of ATP by TAP1. This is a conformational effect, as the ER lumenal domain of US6 is sufficient to inhibit ATP binding by the cytosolic nucleotide binding domain of TAP1. US6 also stabilizes TAP at 37 degrees C and prevents conformational rearrangements induced by peptide binding. Our findings suggest that the association of US6 with TAP stabilizes a conformation in TAP1 that prevents ATP binding and subsequent peptide translocation.
Figures






Similar articles
-
The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP.Immunity. 1997 May;6(5):613-21. doi: 10.1016/s1074-7613(00)80349-0. Immunity. 1997. PMID: 9175839
-
Structural and Functional Dissection of the Human Cytomegalovirus Immune Evasion Protein US6.J Virol. 2008 Apr;82(7):3271-82. doi: 10.1128/JVI.01705-07. Epub 2008 Jan 16. J Virol. 2008. PMID: 18199642 Free PMC article.
-
Molecular mechanism and structural aspects of transporter associated with antigen processing inhibition by the cytomegalovirus protein US6.J Biol Chem. 2001 Dec 21;276(51):48031-9. doi: 10.1074/jbc.M108528200. Epub 2001 Oct 17. J Biol Chem. 2001. PMID: 11606590
-
Spotlight on TAP and its vital role in antigen presentation and cross-presentation.Mol Immunol. 2022 Feb;142:105-119. doi: 10.1016/j.molimm.2021.12.013. Epub 2021 Dec 29. Mol Immunol. 2022. PMID: 34973498 Free PMC article. Review.
-
Function of the transport complex TAP in cellular immune recognition.Biochim Biophys Acta. 1999 Dec 6;1461(2):405-19. doi: 10.1016/s0005-2736(99)00171-6. Biochim Biophys Acta. 1999. PMID: 10581370 Review.
Cited by
-
Equine herpesvirus type 4 UL56 and UL49.5 proteins downregulate cell surface major histocompatibility complex class I expression independently of each other.J Virol. 2012 Aug;86(15):8059-71. doi: 10.1128/JVI.00891-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623773 Free PMC article.
-
Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing.Proc Natl Acad Sci U S A. 2005 Apr 5;102(14):5144-9. doi: 10.1073/pnas.0501463102. Epub 2005 Mar 25. Proc Natl Acad Sci U S A. 2005. PMID: 15793001 Free PMC article.
-
Viral escape mechanisms--escapology taught by viruses.Int J Exp Pathol. 2001 Oct;82(5):269-86. doi: 10.1046/j.1365-2613.2001.00204.x. Int J Exp Pathol. 2001. PMID: 11703537 Free PMC article. Review.
-
Use of chimeric proteins to investigate the role of transporter associated with antigen processing (TAP) structural domains in peptide binding and translocation.Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7241-6. doi: 10.1073/pnas.131132198. Proc Natl Acad Sci U S A. 2001. PMID: 11416206 Free PMC article.
-
Human cytomegalovirus UL18 utilizes US6 for evading the NK and T-cell responses.PLoS Pathog. 2008 Aug 8;4(8):e1000123. doi: 10.1371/journal.ppat.1000123. PLoS Pathog. 2008. PMID: 18688275 Free PMC article.
References
-
- Abele R. and Tampe,R. (1999) Function of the transport complex TAP in cellular immune recognition. Biochim. Biophys. Acta, 1461, 405–419. - PubMed
-
- Ahn K., Gruhler,A., Galocha,B., Jones,T.R., Wiertz,E.J., Ploegh,H.L., Peterson,P.A., Yang,Y. and Fruh,K. (1997) The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity, 6, 613–621. - PubMed
-
- Androlewicz M.J. and Cresswell,P. (1994) Human transporters associated with antigen processing possess a promiscuous peptide-binding site. Immunity, 1, 7–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

