Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans
- PMID: 11157776
- PMCID: PMC312614
- DOI: 10.1101/gad.856001
Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans
Abstract
Cryptococcus neoformans is a leading cause of life-threatening fungal infection in immunocompromised patients. Inositol-phosphoryl ceramide synthase 1 (Ipc1) is a fungus-specific enzyme, encoded by the essential IPC1 gene, that catalyzes the formation of complex sphingolipids and may also regulate the levels of phytoceramide and diacylglycerol. Here, we investigated the functions of this essential gene by modulating its expression in C. neoformans using a galactose-inducible promoter. Down-regulation of IPC1 significantly lowers the expression of certain virulence traits such as melanin pigmentation and, remarkably, impairs pathogenicity of C. neoformans in an established rabbit model. Interestingly, we found that IPC1 down-regulation significantly decreases the intracellular growth of C. neoformans in the J774.16 murine macrophage-like cells. Finally, we studied the effect of IPC1 expression under different stress conditions and found that down-regulation of IPC1 confers a defect on in vitro growth at low pH. Because this environment is similar to that in the phagolysosome of J774.16 macrophage-like cells, our findings indicate that down-regulation of IPC1 confers a growth defect in vivo through a pH-dependent mechanism. In conclusion, our study is the first to define a novel and crucial function of Ipc1 in fungal pathogenesis.
Figures





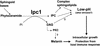
Similar articles
-
APP1 transcription is regulated by inositol-phosphorylceramide synthase 1-diacylglycerol pathway and is controlled by ATF2 transcription factor in Cryptococcus neoformans.J Biol Chem. 2005 Oct 28;280(43):36055-64. doi: 10.1074/jbc.M507285200. Epub 2005 Aug 29. J Biol Chem. 2005. PMID: 16129666
-
The sphingolipid pathway regulates Pkc1 through the formation of diacylglycerol in Cryptococcus neoformans.J Biol Chem. 2004 May 14;279(20):21144-53. doi: 10.1074/jbc.M312995200. Epub 2004 Mar 10. J Biol Chem. 2004. PMID: 15014071
-
The Role of Ceramide Synthases in the Pathogenicity of Cryptococcus neoformans.Cell Rep. 2018 Feb 6;22(6):1392-1400. doi: 10.1016/j.celrep.2018.01.035. Cell Rep. 2018. PMID: 29425496 Free PMC article.
-
The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans.Microbes Infect. 2003 Jun;5(7):667-75. doi: 10.1016/s1286-4579(03)00092-3. Microbes Infect. 2003. PMID: 12787743 Review.
-
Cryptococcus neoformans: virulence and host defences.Med Mycol. 1998;36 Suppl 1:79-86. Med Mycol. 1998. PMID: 9988495 Review.
Cited by
-
Role of phagocytosis in the virulence of Cryptococcus neoformans.Eukaryot Cell. 2004 Oct;3(5):1067-75. doi: 10.1128/EC.3.5.1067-1075.2004. Eukaryot Cell. 2004. PMID: 15470235 Free PMC article. Review. No abstract available.
-
Metabolomics Analysis Identifies Sphingolipids as Key Signaling Moieties in Appressorium Morphogenesis and Function in Magnaporthe oryzae.mBio. 2019 Aug 20;10(4):e01467-19. doi: 10.1128/mBio.01467-19. mBio. 2019. PMID: 31431550 Free PMC article.
-
Characterization of inositol phospho-sphingolipid-phospholipase C 1 (Isc1) in Cryptococcus neoformans reveals unique biochemical features.FEBS Lett. 2011 Feb 18;585(4):635-40. doi: 10.1016/j.febslet.2011.01.015. Epub 2011 Jan 21. FEBS Lett. 2011. PMID: 21256847 Free PMC article.
-
Sphingolipidomics: An Important Mechanistic Tool for Studying Fungal Pathogens.Front Microbiol. 2016 Apr 14;7:501. doi: 10.3389/fmicb.2016.00501. eCollection 2016. Front Microbiol. 2016. PMID: 27148190 Free PMC article. Review.
-
Biosynthesis and immunogenicity of glucosylceramide in Cryptococcus neoformans and other human pathogens.Eukaryot Cell. 2007 Oct;6(10):1715-26. doi: 10.1128/EC.00208-07. Epub 2007 Aug 10. Eukaryot Cell. 2007. PMID: 17693597 Free PMC article. Review. No abstract available.
References
-
- Agin PP, Dowdy JC, Costlow ME. Diacylglycerol-induced melanogenesis in Skh-2 pigmented hair-less mice. Photodermatol Photoimmunol Photomed. 1991;8:51–56. - PubMed
-
- Alspaugh JA, Perfect JR, Heitman J. Signal transduction pathways regulating differentiation and pathogenicity of Cryptococcus neoformans. Fungal Genet Biol. 1998;25:1–14. - PubMed
-
- Barluzzi R, Brozzetti A, Mariucci G, Tantucci M, Neglia RG, Bistoni F, Blasi E. Establishment of protective immunity against cerebral cryptococcosis by means of an avirulent, non melanogenic Cryptococcus neoformansstrain. J Neuroimmunol. 2000;109:75–86. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
