Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus
- PMID: 11157922
- PMCID: PMC94983
- DOI: 10.1128/JB.183.4.1113-1123.2001
Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus
Abstract
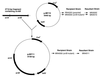
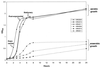
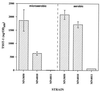
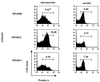
We have previously demonstrated that the presence of oxygen is necessary for the production of toxic shock syndrome toxin 1 (TSST-1) by Staphylococcus aureus in vitro. To investigate the mechanism by which oxygen might regulate toxin production, we identified homologs in S. aureus of the Bacillus subtilis resDE genes. The two-component regulatory system encoded by resDE, ResD-ResE, has been implicated in the global regulation of aerobic and anaerobic respiratory metabolism in B. subtilis. We have designated the S. aureus homologs srrAB (staphylococcal respiratory response). The effects of srrAB expression on expression of RNAIII (the effector molecule of the agr locus) and on production of TSST-1 (an exotoxin) and protein A (a surface-associated virulence factor) were investigated. Expression of RNAIII was inversely related to expression of srrAB. Disruption of srrB resulted in increased levels of RNAIII, while expression of srrAB in trans on a multicopy plasmid resulted in repression of RNAIII transcription, particularly in microaerobic conditions. Disruption of srrB resulted in decreased production of TSST-1 under microaerobic conditions and, to a lesser extent, under aerobic conditions as well. Overexpression of srrAB resulted in nearly complete repression of TSST-1 production in both microaerobic and aerobic conditions. Protein A production by the srrB mutant was upregulated in microaerobic conditions and decreased in aerobic conditions. Protein A production was restored to nearly wild-type levels by complementation of srrAB into the null mutant. These results indicate that the putative two-component system encoded by srrAB, SrrA-SrrB, acts in the global regulation of staphylococcal virulence factors, and may repress virulence factors under low-oxygen conditions. Furthermore, srrAB may provide a mechanistic link between respiratory metabolism, environmental signals, and regulation of virulence factors in S. aureus.
Figures



Similar articles
-
Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus.J Bacteriol. 2004 Apr;186(8):2430-8. doi: 10.1128/JB.186.8.2430-2438.2004. J Bacteriol. 2004. PMID: 15060046 Free PMC article.
-
Repression of Staphylococcus aureus SrrAB using inducible antisense srrA alters growth and virulence factor transcript levels.Biochemistry. 2007 Jan 9;46(1):314-21. doi: 10.1021/bi0603266. Biochemistry. 2007. PMID: 17198402
-
The SrrAB two-component system regulates Staphylococcus aureus pathogenicity through redox sensitive cysteines.Proc Natl Acad Sci U S A. 2020 May 19;117(20):10989-10999. doi: 10.1073/pnas.1921307117. Epub 2020 Apr 30. Proc Natl Acad Sci U S A. 2020. PMID: 32354997 Free PMC article.
-
Regulation of virulence determinants in Staphylococcus aureus.Int J Med Microbiol. 2001 May;291(2):159-70. doi: 10.1078/1438-4221-00112. Int J Med Microbiol. 2001. PMID: 11437338 Review.
-
Regulation of virulence determinants in Staphylococcus aureus: complexity and applications.FEMS Microbiol Rev. 2004 May;28(2):183-200. doi: 10.1016/j.femsre.2003.09.003. FEMS Microbiol Rev. 2004. PMID: 15109784 Review.
Cited by
-
Control of the Staphylococcus aureus toxic shock tst promoter by the global regulator SarA.J Bacteriol. 2010 Nov;192(22):6077-85. doi: 10.1128/JB.00146-10. Epub 2010 Sep 24. J Bacteriol. 2010. PMID: 20870770 Free PMC article.
-
Inactivation of a two-component signal transduction system, SaeRS, eliminates adherence and attenuates virulence of Staphylococcus aureus.Infect Immun. 2006 Aug;74(8):4655-65. doi: 10.1128/IAI.00322-06. Infect Immun. 2006. PMID: 16861653 Free PMC article.
-
A clash of quorum sensing vs quorum sensing inhibitors: an overview and risk of resistance.Arch Microbiol. 2023 Mar 7;205(4):107. doi: 10.1007/s00203-023-03442-x. Arch Microbiol. 2023. PMID: 36881156 Review.
-
nblS, a gene involved in controlling photosynthesis-related gene expression during high light and nutrient stress in Synechococcus elongatus PCC 7942.J Bacteriol. 2002 May;184(9):2481-90. doi: 10.1128/JB.184.9.2481-2490.2002. J Bacteriol. 2002. PMID: 11948163 Free PMC article.
-
The small non-coding RNA RsaE influences extracellular matrix composition in Staphylococcus epidermidis biofilm communities.PLoS Pathog. 2019 Mar 14;15(3):e1007618. doi: 10.1371/journal.ppat.1007618. eCollection 2019 Mar. PLoS Pathog. 2019. PMID: 30870530 Free PMC article.
References
-
- Barrett J F, Goldschmidt R M, Lawrence L E, Foleno B, Chen R, Demers J P, Johnson S, Kanojia R, Fernandez J, Bernstein J, Licata L, Donetz A, Huang S, Hlasta D J, Macielag M J, Ohemeng K, Frechette R, Frosco M B, Klaubert D H, Whiteley J M, Wang L, Hoch J A. Antibacterial agents that inhibit two-component signal transduction systems. Proc Natl Acad Sci USA. 1998;95:5317–5322. - PMC - PubMed
-
- Blomster-Hautamaa D A, Schlievert P M. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:37–43. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

