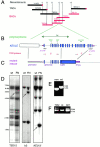
The cytokinesis gene KEULE encodes a Sec1 protein that binds the syntaxin KNOLLE
- PMID: 11157980
- PMCID: PMC2195996
- DOI: 10.1083/jcb.152.3.531
The cytokinesis gene KEULE encodes a Sec1 protein that binds the syntaxin KNOLLE
Abstract
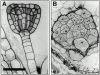
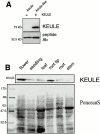
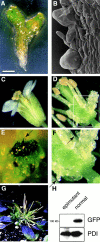
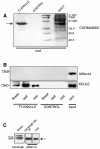
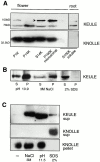
KEULE is required for cytokinesis in Arabidopsis thaliana. We have positionally cloned the KEULE gene and shown that it encodes a Sec1 protein. KEULE is expressed throughout the plant, yet appears enriched in dividing tissues. Cytokinesis-defective mutant sectors were observed in all somatic tissues upon transformation of wild-type plants with a KEULE-green fluorescent protein gene fusion, suggesting that KEULE is required not only during embryogenesis, but at all stages of the plant's life cycle. KEULE is characteristic of a Sec1 protein in that it appears to exist in two forms: soluble or peripherally associated with membranes. More importantly, KEULE binds the cytokinesis-specific syntaxin KNOLLE. Sec1 proteins are key regulators of vesicle trafficking, capable of integrating a large number of intra- and/or intercellular signals. As a cytokinesis-related Sec1 protein, KEULE appears to represent a novel link between cell cycle progression and the membrane fusion apparatus.
Figures


References
-
- Assaad F.F., Tucker K.L., Signer E.R. Epigenetic repeat-induced gene silencing (RIGS) in Arabidopsis . Plant Mol. Biol. 1993;22:1067–1085. - PubMed
-
- Assaad F., Mayer U., Wanner G., Jürgens G. The keule gene is involved in cytokinesis in Arabidopsis . Mol. Gen. Genet. 1996;253:267–277. - PubMed
-
- Assaad F.F., Lukowitz W., Mayer U., Jürgens G. Cytokinesis in somatic plant cells. Plant Physiol. Biochem. 1997;35:175–182.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

