Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis
- PMID: 11157988
- PMCID: PMC2196007
- DOI: 10.1083/jcb.152.3.633
Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis
Abstract
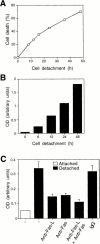
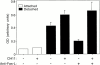
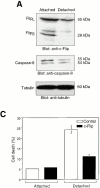
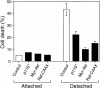
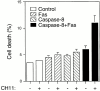
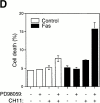
Survival of endothelial cells is critical for cellular processes such as angiogenesis. Cell attachment to extracellular matrix inhibits apoptosis in endothelial cells both in vitro and in vivo, but the molecular mechanisms underlying matrix-induced survival signals or detachment-induced apoptotic signals are unknown. We demonstrate here that matrix attachment is an efficient regulator of Fas-mediated apoptosis in endothelial cells. Thus, matrix attachment protects cells from Fas-induced apoptosis, whereas matrix detachment results in susceptibility to Fas-mediated cell death. Matrix attachment modulates Fas-mediated apoptosis at two different levels: by regulating the expression level of Fas, and by regulating the expression level of c-Flip, an endogenous antagonist of caspase-8. The extracellular signal-regulated kinase (Erk) cascade functions as a survival pathway in adherent cells by regulating c-Flip expression. We further show that detachment-induced cell death, or anoikis, itself results from activation of the Fas pathway by its ligand, Fas-L. Fas-L/Fas interaction, Fas-FADD complex formation, and caspase-8 activation precede the bulk of anoikis in endothelial cells, and inhibition of any of these events blocks anoikis. These studies identify matrix attachment as a survival factor against death receptor-mediated apoptosis and provide a molecular mechanism for anoikis and previously observed Fas resistance in endothelial cells.
Figures




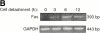
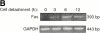





References
-
- Aoudjit F., Vuori K. Engagement of the alpha2beta1 integrin inhibits Fas ligand expression and activation-induced cell death in T cells in a focal adhesion kinase-dependent manner. Blood. 2000;95:2044–2051. - PubMed
-
- Barberis L., Wary K.K., Fiucci G., Liu F., Hirsch E., Brancaccio M., Altruda F., Tarone G., Giancotti F.G. Distinct roles of the adaptor protein shc and focal adhesion kinase in integrin signaling to ERK. J. Biol. Chem. 2000;275:36532–36540. - PubMed
-
- Brooks P.C., Montgomery A.M., Rosenfeld M., Reisfeld R.A., Hu T., Klier G., Cheresh D.A. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. - PubMed
-
- Datta S.R., Brunet A., Greenberg M.E. Cellular survivala play in three Akts. Genes Dev. 1999;13:2905–2927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

