A Shared noncapsular antigen is responsible for false-positive reactions by Staphylococcus epidermidis in commercial agglutination tests for Staphylococcus aureus
- PMID: 11158104
- PMCID: PMC87773
- DOI: 10.1128/JCM.39.2.544-550.2001
A Shared noncapsular antigen is responsible for false-positive reactions by Staphylococcus epidermidis in commercial agglutination tests for Staphylococcus aureus
Abstract
Many of the commercial slide agglutination tests for Staphylococcus aureus incorporate antibodies against cell surface antigens associated with methicillin resistance, including capsular polysaccharides and an uncharacterized antigen, serotype 18. These tests are more sensitive than the first-generation agglutination procedures that detected only bound coagulase and protein A, but they suffer from false-positive reactions with some coagulase-negative staphylococci. The aim of this study was to elucidate the mechanism for false-positive agglutination by S. epidermidis in these tests. A group of methicillin-resistant S. aureus (MRSA) isolates, including a serotype 18 strain, that were not detectable in the first-generation tests were found to be of capsular polysaccharide type 8. All of these isolates were deficient in bound coagulase and/or protein A, and they possessed a heat-stable, proteinaceous antigen that was absent from a prototype capsule type 8 strain. Enzyme-linked immunosorbent assay and agarose gel immunodiffusion experiments demonstrated that this proteinaceous antigen was also present on both methicillin-sensitive and methicillin-resistant S. epidermidis clinical isolates. S. epidermidis strains that gave false-positive agglutination test results had a considerably higher level of this antigen than strains that gave the correct negative result. These findings reveal the importance of the careful selection of MRSA strains for raising anti-capsular type 8 antibodies for use in agglutination tests. Strains devoid of the antigen shared with S. epidermidis should be used to eliminate potential cross-reactions with this coagulase-negative coccus.
Figures
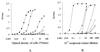
 ).
).

References
-
- Bignardi G E, Woodford N, Chapman A, Johnson A P, Speller D C E. Detection of the mec-A gene and phenotypic detection of resistance in Staphylococcus aureus isolates with borderline or low-level methicillin resistance. J Antimicrob Chemother. 1996;37:53–63. - PubMed
-
- Chabbert Y A, Pillet J. Correlation between methicillin resistance and serotype in Staphylococcus. Nature. 1967;213:1137. - PubMed
-
- Christensen G D, Simpson W A, Younger J J, Baddour L M, Barrett F F, Melton D M, Beachey E H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

