Cooperative mechanisms in the activation dependence of the rate of force development in rabbit skinned skeletal muscle fibers
- PMID: 11158166
- PMCID: PMC2217243
- DOI: 10.1085/jgp.117.2.133
Cooperative mechanisms in the activation dependence of the rate of force development in rabbit skinned skeletal muscle fibers
Abstract
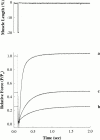
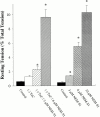
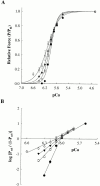
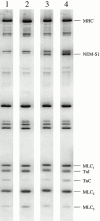
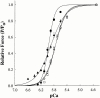
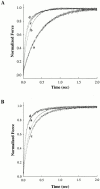
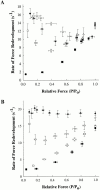
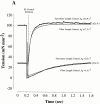
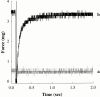
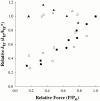
Regulation of contraction in skeletal muscle is a highly cooperative process involving Ca(2+) binding to troponin C (TnC) and strong binding of myosin cross-bridges to actin. To further investigate the role(s) of cooperation in activating the kinetics of cross-bridge cycling, we measured the Ca(2+) dependence of the rate constant of force redevelopment (k(tr)) in skinned single fibers in which cross-bridge and Ca(2+) binding were also perturbed. Ca(2+) sensitivity of tension, the steepness of the force-pCa relationship, and Ca(2+) dependence of k(tr) were measured in skinned fibers that were (1) treated with NEM-S1, a strong-binding, non-force-generating derivative of myosin subfragment 1, to promote cooperative strong binding of endogenous cross-bridges to actin; (2) subjected to partial extraction of TnC to disrupt the spread of activation along the thin filament; or (3) both, partial extraction of TnC and treatment with NEM-S1. The steepness of the force-pCa relationship was consistently reduced by treatment with NEM-S1, by partial extraction of TnC, or by a combination of TnC extraction and NEM-S1, indicating a decrease in the apparent cooperativity of activation. Partial extraction of TnC or NEM-S1 treatment accelerated the rate of force redevelopment at each submaximal force, but had no effect on kinetics of force development in maximally activated preparations. At low levels of Ca(2+), 3 microM NEM-S1 increased k(tr) to maximal values, and higher concentrations of NEM-S1 (6 or 10 microM) increased k(tr) to greater than maximal values. NEM-S1 also accelerated k(tr) at intermediate levels of activation, but to values that were submaximal. However, the combination of partial TnC extraction and 6 microM NEM-S1 increased k(tr) to virtually identical supramaximal values at all levels of activation, thus, completely eliminating the activation dependence of k(tr). These results show that k(tr) is not maximal in control fibers, even at saturating [Ca(2+)], and suggest that activation dependence of k(tr) is due to the combined activating effects of Ca(2+) binding to TnC and cross-bridge binding to actin.
Figures


Similar articles
-
Effect of Ca2+ binding properties of troponin C on rate of skeletal muscle force redevelopment.Am J Physiol Cell Physiol. 2010 Nov;299(5):C1091-9. doi: 10.1152/ajpcell.00491.2009. Epub 2010 Aug 11. Am J Physiol Cell Physiol. 2010. PMID: 20702687 Free PMC article.
-
Strong binding of myosin increases shortening velocity of rabbit skinned skeletal muscle fibres at low levels of Ca(2+).J Physiol. 2001 Jun 1;533(Pt 2):357-65. doi: 10.1111/j.1469-7793.2001.0357a.x. J Physiol. 2001. PMID: 11389197 Free PMC article.
-
Regulation of force development studied by photolysis of caged ADP in rabbit skinned psoas fibers.Biophys J. 2001 Jul;81(1):334-44. doi: 10.1016/S0006-3495(01)75703-7. Biophys J. 2001. PMID: 11423418 Free PMC article.
-
Regulation of contraction in striated muscle.Physiol Rev. 2000 Apr;80(2):853-924. doi: 10.1152/physrev.2000.80.2.853. Physiol Rev. 2000. PMID: 10747208 Review.
-
Cross-bridges affect both TnC structure and calcium affinity in muscle fibers.Adv Exp Med Biol. 1993;332:183-92; discussion 192-4. doi: 10.1007/978-1-4615-2872-2_17. Adv Exp Med Biol. 1993. PMID: 8109332 Review.
Cited by
-
Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres.J Physiol. 2010 Sep 15;588(Pt 18):3567-92. doi: 10.1113/jphysiol.2010.188508. Epub 2010 Jul 26. J Physiol. 2010. PMID: 20660569 Free PMC article.
-
Effect of Ca2+ binding properties of troponin C on rate of skeletal muscle force redevelopment.Am J Physiol Cell Physiol. 2010 Nov;299(5):C1091-9. doi: 10.1152/ajpcell.00491.2009. Epub 2010 Aug 11. Am J Physiol Cell Physiol. 2010. PMID: 20702687 Free PMC article.
-
Recovery of left ventricular function following in vivo reexpression of cardiac myosin binding protein C.J Gen Physiol. 2019 Jan 7;151(1):77-89. doi: 10.1085/jgp.201812238. Epub 2018 Dec 20. J Gen Physiol. 2019. PMID: 30573635 Free PMC article.
-
Divergent effects of α- and β-myosin heavy chain isoforms on the N terminus of rat cardiac troponin T.J Gen Physiol. 2013 Oct;142(4):413-23. doi: 10.1085/jgp.201310971. Epub 2013 Sep 16. J Gen Physiol. 2013. PMID: 24043862 Free PMC article.
-
A Spatially Detailed Model of Isometric Contraction Based on Competitive Binding of Troponin I Explains Cooperative Interactions between Tropomyosin and Crossbridges.PLoS Comput Biol. 2015 Aug 11;11(8):e1004376. doi: 10.1371/journal.pcbi.1004376. eCollection 2015 Aug. PLoS Comput Biol. 2015. PMID: 26262582 Free PMC article.
References
-
- Brandt P.W., Diamond M.S., Schachat F.H. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. J. Mol. Biol. 1984;180:379–384. - PubMed
-
- Brandt P.W., Roemer D., Schachat F.H. Cooperative activation of skeletal muscle thin filaments by rigor crossbridgesthe effect of troponin C extraction. J. Mol. Biol. 1990;212:473–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

