Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat
- PMID: 11158274
- PMCID: PMC2278417
- DOI: 10.1111/j.1469-7793.2001.0431k.x
Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat
Retraction in
-
Retraction: Zhu, J. X., Wu, X. Y., Owyang, C. & Li, Y. (2001). Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. The Journal of Physiology, 530(3), 431-442. https://doi.org/10.1111/j.1469-7793.2001.0431k.x.J Physiol. 2023 May;601(10):2047. doi: 10.1113/JP284695. Epub 2023 Apr 28. J Physiol. 2023. PMID: 36942647 No abstract available.
Abstract
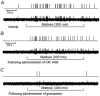
The vagus nerve conveys primary afferent information produced by a meal to the brainstem. Serotonin (5-HT), which abounds in intestinal enterochromaffin cells, is released in response to various stimuli. We have recently demonstrated that 5-HT released from intestinal enterochromaffin cells activates 5-HT3 receptors on vagal afferent fibres to mediate luminal non-cholecystokinin-stimulated pancreatic secretion. The present study was designed to evaluate the responses of vagal sensory neurons to intraluminal osmotic stimulation and luminal infusion of maltose, glucose or 5-HT. We investigated the role of endogenous 5-HT in signal transmission evoked by luminal stimuli to activate vagal sensory neurons. The discharges of vagal primary afferent neurons innervating the intestine were recorded from rat nodose ganglia. Luminal factors such as intestinal osmotic stimuli and perfusion of carbohydrates elicited powerful vagal nodose responses. Electrical subdiaphragmatic vagal stimulation activated 364 single units; 40 of these responded to intestinal mucosal stimuli. Of these 40, 30 responded to intraduodenal perfusion of hyperosmolar NaCl (500 mosmol l(-1)), 27 responded to tap water (5 mosmol l(-1)) and 20 and 19 responded to maltose (300 mM) and glucose (277.5 mM), respectively. The 5-HT3/4 antagonist tropisetron (ICS 205-930) or 5-HT3 antagonist granisetron abolished luminal stimuli-evoked nodose neuronal responses. Intraluminal infusion of 10(-5) and 10(-4) M 5-HT elicited increases in vagal afferent discharge in 25 and 31 units, respectively, by activating the 5-HT3 receptors. Acute subdiaphragmatic vagotomy, intestinal mucosal application of the local anaesthetic lidocaine (lignocaine) or administration of 5-HT3 antagonist each abolished the luminal 5-HT-induced nodose neuronal responses. In contrast, distension-sensitive neurons did not respond to duodenal infusion of 5-HT. Pharmacological depletion of 5-HT stores using p-chlorophenylalanine (PCPA), a 5-HT-synthesis inhibitor, abolished luminal factor-stimulated nodose neuronal responses. In contrast, pretreatment with 5,7-dihydroxytryptamine (5,7-DHT), a specific 5-HT neurotoxin that destroys 5-HT-containing neurons without affecting 5-HT-containing mucosal cells, had no effect on these responses. These results suggested that the nodose neuronal responses to luminal osmolarity and to the digestion products of carbohydrates are dependent on the release of endogenous 5-HT from the mucosal enterochromaffin cells, which acts on the 5-HT3 receptors on vagal afferent fibres to stimulate vagal sensory neurons.
Figures




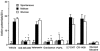
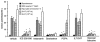


Comment in
-
Primary afferent response to signals in the intestinal lumen.J Physiol. 2001 Feb 1;530(Pt 3):343. doi: 10.1111/j.1469-7793.2001.0343k.x. J Physiol. 2001. PMID: 11158267 Free PMC article.
References
-
- Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Canadian Journal of Physiology and Pharmacology. 1990;68:325–345. - PubMed
-
- Andrews PLR, Davison JS. Activation of vagal afferent terminals by 5-HT is mediated by 5-HT3 receptors in the anaesthetized ferret. Journal of Physiology. 1990;422:92.
-
- Berthoud HR, Kressel M, Raybould HE, Nuehuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo Dil-tracing. Anatomy and Embryology. 1995;191:203–212. - PubMed
-
- Bjorkland A, Baumgarten HG, Rensch A. 5,7-Dihydroxytryptamine: improvement of its selectivity for serotonin neurons in the CNS by pretreatment with desipramine. Journal of Neurochemistry. 1975;24:833–835. - PubMed
-
- Blackshaw LA, Grundy D. Effects of 5-hydroxy-tryptamine on discharge of vagal mucosal receptors from the upper gastrointestinal tract of the ferret. Journal of the Autonomic Nervous System. 1993;45:41–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

