The control of single-celled cotton fiber elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and K+ transporters and expansin
- PMID: 11158528
- PMCID: PMC102212
- DOI: 10.1105/tpc.13.1.47
The control of single-celled cotton fiber elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and K+ transporters and expansin
Abstract
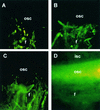
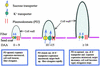
Each cotton fiber is a single cell that elongates to 2.5 to 3.0 cm from the seed coat epidermis within approximately 16 days after anthesis (DAA). To elucidate the mechanisms controlling this rapid elongation, we studied the gating of fiber plasmodesmata and the expression of the cell wall-loosening gene expansin and plasma membrane transporters for sucrose and K(+), the major osmotic solutes imported into fibers. Confocal imaging of the membrane-impermeant fluorescent solute carboxyfluorescein (CF) revealed that the fiber plasmodesmata were initially permeable to CF (0 to 9 DAA), but closed at approximately 10 DAA and re-opened at 16 DAA. A developmental switch from simple to branched plasmodesmata was also observed in fibers at 10 DAA. Coincident with the transient closure of the plasmodesmata, the sucrose and K(+) transporter genes were expressed maximally in fibers at 10 DAA with sucrose transporter proteins predominately localized at the fiber base. Consequently, fiber osmotic and turgor potentials were elevated, driving the rapid phase of elongation. The level of expansin mRNA, however, was high at the early phase of elongation (6 to 8 DAA) and decreased rapidly afterwards. The fiber turgor was similar to the underlying seed coat cells at 6 to 10 DAA and after 16 DAA. These results suggest that fiber elongation is initially achieved largely by cell wall loosening and finally terminated by increased wall rigidity and loss of higher turgor. To our knowledge, this study provides an unprecedented demonstration that the gating of plasmodesmata in a given cell is developmentally reversible and is coordinated with the expression of solute transporters and the cell wall-loosening gene. This integration of plasmodesmatal gating and gene expression appears to control fiber cell elongation.
Figures





References
-
- Basra, A., and Malik, C.P. (1984). Development of the cotton fiber. Int. Rev. Cytol. 89 65–113.
-
- Beffagna, N., and Romani, G. (1988). Effects of two plasmalemma ATPase inhibitors on H+ extrusion and intracellular pH in Elodea densa leaves. J. Exp. Bot. 39 1039–1043.
-
- Buchala, A.J. (1987). Acid β-fructofuranoside fructohydrolase (invertase) in developing cotton (Gossypium arboreum L.) fibers and its relationship to β-glucan synthesis from sucrose fed to the fiber apoplast. J. Plant Physiol. 127 219–230.
-
- Cassman, K.G., Kerby, T.A., Roberts, B.A., Bryant, D.C., and Higashi, S.L. (1990). Potassium nutrition effects on lint yield and fiber quality of Acala cotton. Crop Sci. 30 672–677.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

