Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate
- PMID: 11158538
- PMCID: PMC102208
Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate
Abstract
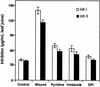
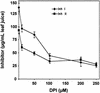
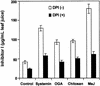
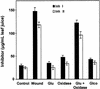
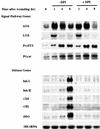
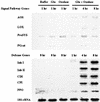
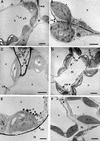
The systemic accumulation of both hydrogen peroxide (H(2)O(2)) and proteinase inhibitor proteins in tomato leaves in response to wounding was inhibited by the NADPH oxidase inhibitors diphenylene iodonium (DPI), imidazole, and pyridine. The expression of several defense genes in response to wounding, systemin, oligosaccharides, and methyl jasmonate also was inhibited by DPI. These genes, including those of four proteinase inhibitors and polyphenol oxidase, are expressed within 4 to 12 hr after wounding. However, DPI did not inhibit the wound-inducible expression of genes encoding prosystemin, lipoxygenase, and allene oxide synthase, which are associated with the octadecanoid signaling pathway and are expressed 0.5 to 2 hr after wounding. Accordingly, treatment of plants with the H(2)O(2)-generating enzyme glucose oxidase plus glucose resulted in the induction of only the later-expressed defensive genes and not the early-expressed signaling-related genes. H(2)O(2) was cytochemically detected in the cell walls of vascular parenchyma cells and spongy mesophyll cells within 4 hr after wounding of wild-type tomato leaves, but not earlier. The cumulative results suggest that active oxygen species are generated near cell walls of vascular bundle cells by oligogalacturonide fragments produced by wound-inducible polygalacturonase and that the resulting H(2)O(2) acts as a second messenger for the activation of defense genes in mesophyll cells. These data provide a rationale for the sequential, coordinated, and functional roles of systemin, jasmonic acid, oligogalacturonides, and H(2)O(2) signals for systemic signaling in tomato plants in response to wounding.
Figures

Similar articles
-
Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway.Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):407-11. doi: 10.1073/pnas.92.2.407. Proc Natl Acad Sci U S A. 1995. PMID: 7831300 Free PMC article.
-
The tomato mutant spr1 is defective in systemin perception and the production of a systemic wound signal for defense gene expression.Plant J. 2003 Feb;33(3):567-76. doi: 10.1046/j.1365-313x.2003.01646.x. Plant J. 2003. PMID: 12581314
-
A gene encoding a chloroplast-targeted lipoxygenase in tomato leaves is transiently induced by wounding, systemin, and methyl jasmonate.Plant Physiol. 1997 Jul;114(3):1085-93. doi: 10.1104/pp.114.3.1085. Plant Physiol. 1997. PMID: 9232884 Free PMC article.
-
Systemin/Jasmonate-mediated systemic defense signaling in tomato.Mol Plant. 2011 Jul;4(4):607-15. doi: 10.1093/mp/ssr008. Epub 2011 Feb 28. Mol Plant. 2011. PMID: 21357647 Review.
-
Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals.Proc Natl Acad Sci U S A. 1996 Oct 29;93(22):12053-8. doi: 10.1073/pnas.93.22.12053. Proc Natl Acad Sci U S A. 1996. PMID: 8901530 Free PMC article. Review.
Cited by
-
A review of the "Omics" approach to biomarkers of oxidative stress in Oryza sativa.Int J Mol Sci. 2013 Apr 8;14(4):7515-41. doi: 10.3390/ijms14047515. Int J Mol Sci. 2013. PMID: 23567269 Free PMC article. Review.
-
JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis.Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10768-E10777. doi: 10.1073/pnas.1811828115. Epub 2018 Oct 22. Proc Natl Acad Sci U S A. 2018. PMID: 30348775 Free PMC article.
-
ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize.J Exp Bot. 2010 Oct;61(15):4399-411. doi: 10.1093/jxb/erq243. Epub 2010 Aug 6. J Exp Bot. 2010. PMID: 20693409 Free PMC article.
-
Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension.Plant Physiol. 2002 Aug;129(4):1627-32. doi: 10.1104/pp.001222. Plant Physiol. 2002. PMID: 12177475 Free PMC article.
-
Identification of expression profiles of sorghum genes in response to greenbug phloem-feeding using cDNA subtraction and microarray analysis.Planta. 2006 Apr;223(5):932-47. doi: 10.1007/s00425-005-0148-1. Epub 2005 Nov 15. Planta. 2006. PMID: 16292568
References
-
- Alvarez, M.E., Penell, R.I., Meijer, P.-J., Ishikawa, A., Dixon, R.A., and Lamb, C. (1998). Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92 773–784. - PubMed
-
- Angelini, R., Manes, F., and Federico, R. (1990). Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 182 89–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
