Protein-protein interactions with subunits of human nuclear RNase P
- PMID: 11158571
- PMCID: PMC14685
- DOI: 10.1073/pnas.98.3.920
Protein-protein interactions with subunits of human nuclear RNase P
Abstract
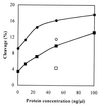
A yeast two-hybrid system was used to analyze interactions among the protein subunits of human nuclear RNase P themselves and with other interacting partners encoded in a HeLa cell cDNA library. Subunits hpop1, Rpp21, Rpp29, Rpp30, Rpp38, and Rpp40 are involved in extensive, but weak, protein-protein interactions in the holoenzyme complex. Rpp14, Rpp20, and Rpp30 were found to have strong interactions with proteins encoded in the cDNA library. The small heat shock protein 27, which interacts with Rpp20 in the two-hybrid assay, binds to Rpp20 during affinity chromatography and can be found to be associated with, and enhances the activity of, highly purified RNase P. RNase P activity in HeLa cell nuclei also increases under the stress of heat shock.
Figures


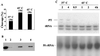

Similar articles
-
Rpp14 and Rpp29, two protein subunits of human ribonuclease P.RNA. 1999 Feb;5(2):153-7. doi: 10.1017/s135583829800185x. RNA. 1999. PMID: 10024167 Free PMC article.
-
Protein-RNA interactions in the subunits of human nuclear RNase P.RNA. 2001 Jul;7(7):937-41. doi: 10.1017/s1355838201010299. RNA. 2001. PMID: 11455963 Free PMC article.
-
Purification and characterization of Rpp25, an RNA-binding protein subunit of human ribonuclease P.RNA. 2002 Mar;8(3):290-5. doi: 10.1017/s1355838202027954. RNA. 2002. PMID: 12003489 Free PMC article.
-
Structure-function analysis in nuclear RNase P RNA.Mol Biol Rep. 1995-1996;22(2-3):157-60. doi: 10.1007/BF00988722. Mol Biol Rep. 1995. PMID: 8901504 Review.
-
Human ribonuclease P: subunits, function, and intranuclear localization.RNA. 2002 Jan;8(1):1-7. doi: 10.1017/s1355838202011184. RNA. 2002. PMID: 11871657 Free PMC article. Review.
Cited by
-
Inventory and analysis of the protein subunits of the ribonucleases P and MRP provides further evidence of homology between the yeast and human enzymes.Nucleic Acids Res. 2006;34(18):5145-56. doi: 10.1093/nar/gkl626. Epub 2006 Sep 22. Nucleic Acids Res. 2006. PMID: 16998185 Free PMC article.
-
RNA binding protein POP7 regulates ILF3 mRNA stability and expression to promote breast cancer progression.Cancer Sci. 2022 Nov;113(11):3801-3813. doi: 10.1111/cas.15430. Epub 2022 Sep 6. Cancer Sci. 2022. PMID: 35579257 Free PMC article.
-
Structure of Pfu Pop5, an archaeal RNase P protein.Proc Natl Acad Sci U S A. 2006 Jan 24;103(4):873-8. doi: 10.1073/pnas.0508004103. Epub 2006 Jan 17. Proc Natl Acad Sci U S A. 2006. PMID: 16418270 Free PMC article.
-
Heterodimerization regulates RNase MRP/RNase P association, localization, and expression of Rpp20 and Rpp25.RNA. 2007 Jan;13(1):65-75. doi: 10.1261/rna.237807. Epub 2006 Nov 21. RNA. 2007. PMID: 17119099 Free PMC article.
-
Ribonuclease P: the evolution of an ancient RNA enzyme.Crit Rev Biochem Mol Biol. 2006 Mar-Apr;41(2):77-102. doi: 10.1080/10409230600602634. Crit Rev Biochem Mol Biol. 2006. PMID: 16595295 Free PMC article.
References
-
- Altman S, Kirsebom L. In: The RNA World. 2nd Ed. Gesteland R F, Cech T-R, Atkins J F, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 351–380.
-
- Eenennaam H V, Jarrous N, Venrooij W, J, Pruijn G J M. IUBMB Life. 2000;49:265–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

