Caspase-2 pre-mRNA alternative splicing: Identification of an intronic element containing a decoy 3' acceptor site
- PMID: 11158574
- PMCID: PMC14688
- DOI: 10.1073/pnas.98.3.938
Caspase-2 pre-mRNA alternative splicing: Identification of an intronic element containing a decoy 3' acceptor site
Abstract
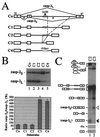
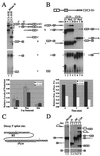
We have established a model system using the caspase-2 pre-mRNA and initiated a study on the role of alternative splicing in regulation of programmed cell death. A caspase-2 minigene construct has been made that can be alternatively spliced in transfected cells and in nuclear extracts. Using this system, we have identified a 100-nt region in downstream intron 9 that inhibits the inclusion of the 61-bp alternative exon. This element (In100) can facilitate exon skipping in the context of competing 3' or 5' splice sites, but not in single-intron splicing units. The In100 element is also active in certain heterologous pre-mRNAs, although in a highly context-dependent manner. Interestingly, we found that In100 contains a sequence that highly resembles a bona fide 3' splice site. We provide evidence that this sequence acts as a "decoy" acceptor site that engages in U2 snRNP-dependent but nonproductive splicing complexes with the 5' splice site of exon 9, hence conferring competitive advantage to the exon-skipping splicing event (E8-E10). These results reveal a mechanism of action for a negative intronic regulatory element and uncover a role for U2 snRNP in the regulation of alternative splicing.
Figures



Similar articles
-
Polypyrimidine track-binding protein binding downstream of caspase-2 alternative exon 9 represses its inclusion.J Biol Chem. 2001 Mar 16;276(11):8535-43. doi: 10.1074/jbc.M008924200. Epub 2000 Dec 14. J Biol Chem. 2001. PMID: 11116151 Free PMC article.
-
An intronic polypyrimidine-rich element downstream of the donor site modulates cystic fibrosis transmembrane conductance regulator exon 9 alternative splicing.J Biol Chem. 2004 Apr 23;279(17):16980-8. doi: 10.1074/jbc.M313439200. Epub 2004 Feb 13. J Biol Chem. 2004. PMID: 14966131
-
An intron element modulating 5' splice site selection in the hnRNP A1 pre-mRNA interacts with hnRNP A1.Mol Cell Biol. 1997 Apr;17(4):1776-86. doi: 10.1128/MCB.17.4.1776. Mol Cell Biol. 1997. PMID: 9121425 Free PMC article.
-
Principles and correction of 5'-splice site selection.RNA Biol. 2022 Jan;19(1):943-960. doi: 10.1080/15476286.2022.2100971. RNA Biol. 2022. PMID: 35866748 Free PMC article. Review.
-
Branch site recognition by the spliceosome.RNA. 2024 Oct 16;30(11):1397-1407. doi: 10.1261/rna.080198.124. RNA. 2024. PMID: 39187383 Free PMC article. Review.
Cited by
-
An important class of intron retention events in human erythroblasts is regulated by cryptic exons proposed to function as splicing decoys.RNA. 2018 Sep;24(9):1255-1265. doi: 10.1261/rna.066951.118. Epub 2018 Jun 29. RNA. 2018. PMID: 29959282 Free PMC article.
-
Finding signals that regulate alternative splicing in the post-genomic era.Genome Biol. 2002 Oct 23;3(11):reviews0008. doi: 10.1186/gb-2002-3-11-reviews0008. Epub 2002 Oct 23. Genome Biol. 2002. PMID: 12429065 Free PMC article. Review.
-
A dynamic intron retention program enriched in RNA processing genes regulates gene expression during terminal erythropoiesis.Nucleic Acids Res. 2016 Jan 29;44(2):838-51. doi: 10.1093/nar/gkv1168. Epub 2015 Nov 3. Nucleic Acids Res. 2016. PMID: 26531823 Free PMC article.
-
Mutations in tau gene exon 10 associated with FTDP-17 alter the activity of an exonic splicing enhancer to interact with Tra2 beta.J Biol Chem. 2003 May 23;278(21):18997-9007. doi: 10.1074/jbc.M301800200. Epub 2003 Mar 20. J Biol Chem. 2003. PMID: 12649279 Free PMC article.
-
TRAIL-beta and TRAIL-gamma: two novel splice variants of the human TNF-related apoptosis-inducing ligand (TRAIL) without apoptotic potential.Br J Cancer. 2003 Mar 24;88(6):918-27. doi: 10.1038/sj.bjc.6600772. Br J Cancer. 2003. PMID: 12644830 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

