Characterization of Rny1, the Saccharomyces cerevisiae member of the T2 RNase family of RNases: unexpected functions for ancient enzymes?
- PMID: 11158587
- PMCID: PMC14701
- DOI: 10.1073/pnas.98.3.1018
Characterization of Rny1, the Saccharomyces cerevisiae member of the T2 RNase family of RNases: unexpected functions for ancient enzymes?
Abstract
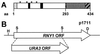
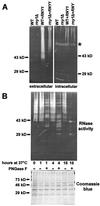
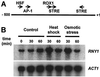
The T(2) family of nonspecific endoribonucleases (EC ) is a widespread family of RNases found in every organism examined thus far. Most T(2) enzymes are secretory RNases and therefore are found extracellularly or in compartments of the endomembrane system that would minimize their contact with cellular RNA. Although the biological functions of various T(2) RNases have been postulated on the basis of enzyme location or gene expression patterns, the cellular roles of these enzymes are generally unknown. In the present work, we characterized Rny1, the only T(2) RNase in Saccharomyces cerevisiae. Rny1 was found to be an active, secreted RNase whose gene expression is controlled by heat shock and osmotic stress. Inactivation of RNY1 leads to unusually large cells that are temperature-sensitive for growth. These phenotypes can be complemented not only by RNY1 but also by both structurally related and unrelated secretory RNases. Additionally, the complementation depends on RNase activity. When coupled with a recent report on the effect of specific RNAs on membrane permeability [Khvorova, A., Kwak, Y-G., Tamkun, M., Majerfeld, I. & Yarus, M. (1999) Proc. Natl. Acad. Sci. USA 96, 10649-10654], our work suggests an unexpected role for Rny1 and possibly other secretory RNases. These enzymes may regulate membrane permeability or stability, a hypothesis that could present an alternative perspective for understanding their functions.
Figures

Similar articles
-
A novel family of RNA tetraloop structure forms the recognition site for Saccharomyces cerevisiae RNase III.EMBO J. 2001 Dec 17;20(24):7240-9. doi: 10.1093/emboj/20.24.7240. EMBO J. 2001. PMID: 11743000 Free PMC article.
-
NnSR1, a class III non-S-RNase constitutively expressed in styles, is induced in roots and stems under phosphate deficiency in Nicotiana alata.Ann Bot. 2013 Nov;112(7):1351-60. doi: 10.1093/aob/mct207. Epub 2013 Sep 18. Ann Bot. 2013. PMID: 24047716 Free PMC article.
-
Ribonucleases from T2 family.Crit Rev Microbiol. 2002;28(2):79-122. doi: 10.1080/1040-840291046704. Crit Rev Microbiol. 2002. PMID: 12109772 Review.
-
Specific binding of a Pop6/Pop7 heterodimer to the P3 stem of the yeast RNase MRP and RNase P RNAs.RNA. 2007 Oct;13(10):1648-55. doi: 10.1261/rna.654407. Epub 2007 Aug 23. RNA. 2007. PMID: 17717080 Free PMC article.
-
The role of mammalian ribonucleases (RNases) in cancer.Biochim Biophys Acta. 2009 Dec;1796(2):99-113. doi: 10.1016/j.bbcan.2009.05.002. Epub 2009 May 20. Biochim Biophys Acta. 2009. PMID: 19463900 Review.
Cited by
-
Inhibition of Enveloped Virus Surrogate Phi6 Infection Using Yeast-Derived Vacuoles.Microbiol Spectr. 2023 Feb 14;11(1):e0266122. doi: 10.1128/spectrum.02661-22. Epub 2023 Jan 23. Microbiol Spectr. 2023. PMID: 36688634 Free PMC article.
-
Visualization of membrane RNAs.RNA. 2003 Nov;9(11):1353-61. doi: 10.1261/rna.5129803. RNA. 2003. PMID: 14561885 Free PMC article.
-
Stress-induced RNASET2 overexpression mediates melanocyte apoptosis via the TRAF2 pathway in vitro.Cell Death Dis. 2014 Jan 23;5(1):e1022. doi: 10.1038/cddis.2013.539. Cell Death Dis. 2014. PMID: 24457966 Free PMC article.
-
RNase T2 genes from rice and the evolution of secretory ribonucleases in plants.Mol Genet Genomics. 2010 Apr;283(4):381-96. doi: 10.1007/s00438-010-0524-9. Epub 2010 Feb 25. Mol Genet Genomics. 2010. PMID: 20182746
-
The Ins and Outs of Autophagic Ribosome Turnover.Cells. 2019 Dec 10;8(12):1603. doi: 10.3390/cells8121603. Cells. 2019. PMID: 31835634 Free PMC article. Review.
References
-
- Benner S A, Ciglic M I, Haugg M, Jermann T M, Opitz J G, Raillard-Yoon S-A, Soucek J, Stackhouse J, Trabesinger-Rüf N, Trautwein K, et al. In: Ribonucleases: Structures and Functions. D'Alessio G, Riordan J S, editors. New York: Academic; 1997. pp. 213–243.
-
- Cuchillo C M, Vilanova M, Nogués V. In: Ribonucleases: Structures and Functions. D'Alessio G, Riordan J S, editors. New York: Academic; 1997. pp. 271–304.
-
- Irie M. In: Ribonucleases: Structures and Functions. D'Alessio G, Riordan J S, editors. New York: Academic; 1997. pp. 101–130.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

