A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors
- PMID: 11158604
- PMCID: PMC14718
- DOI: 10.1073/pnas.98.3.1118
A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors
Abstract
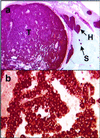
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant cancer syndrome, characterized primarily by multiple tumors in the parathyroid glands, endocrine pancreas, and anterior pituitary. Other tumors, including gastrinoma, carcinoid, adrenal cortical tumors, angiofibroma, collagenoma, and lipoma, also occur in some patients. Individuals with MEN1 almost always have loss-of-function mutations in the MEN1 gene on chromosome 11, and endocrine tumors arising in these patients usually show somatic loss of the remaining wild-type allele. To examine the role of MEN1 in tumor formation, a mouse model was generated through homologous recombination of the mouse homolog Men1. Homozygous mice die in utero at embryonic days 11.5-12.5, whereas heterozygous mice develop features remarkably similar to those of the human disorder. As early as 9 months, pancreatic islets show a range of lesions from hyperplasia to insulin-producing islet cell tumors, and parathyroid adenomas are also frequently observed. Larger, more numerous tumors involving pancreatic islets, parathyroids, thyroid, adrenal cortex, and pituitary are seen by 16 months. All of the tumors tested to date show loss of the wild-type Men1 allele, further supporting its role as a tumor suppressor gene.
Figures



References
-
- Trump D, Farren B, Wooding C, Pang J T, Besser G M, Buchanan K D, Edwards C R, Heath D A, Jackson C E, Jansen S, et al. Q J Med. 1996;89:653–669. - PubMed
-
- Marx S. In: Genetic Basis of Human Cancer. Vogelstein B, Kinzler K, editors. New York: McGraw–Hill; 1998. pp. 489–506.
-
- Larsson C, Skogseid B, Oberg K, Nakamura Y, Nordenskjold M. Nature (London) 1988;332:85–87. - PubMed
-
- Chandrasekharappa S C, Guru S C, Manickam P, Olufemi S E, Collins F S, Emmert-Buck M R, Debelenko L V, Zhuang Z, Lubensky I A, Liotta L A, et al. Science. 1997;276:404–407. - PubMed
-
- Agarwal S K, Kester M B, Debelenko L V, Heppner C, Emmert-Buck M R, Skarulis M C, Doppman J L, Kim Y S, Lubensky I A, Zhuang Z, et al. Hum Mol Genet. 1997;6:1169–1175. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

