Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase 1
- PMID: 11158615
- PMCID: PMC14730
- DOI: 10.1073/pnas.98.3.1188
Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase 1
Abstract
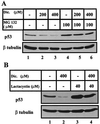
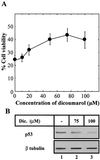
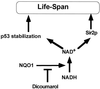
The tumor suppressor gene wild-type p53 encodes a labile protein that accumulates in cells after different stress signals and can cause either growth arrest or apoptosis. One of the p53 target genes, p53-inducible gene 3 (PIG3), encodes a protein with significant homology to oxidoreductases, enzymes involved in cellular responses to oxidative stress and irradiation. This fact raised the possibility that cellular oxidation-reduction events controlled by such enzymes also may regulate the level of p53. Here we show that NADH quinone oxidoreductase 1 (NQO1) regulates p53 stability. The NQO1 inhibitor dicoumarol caused a reduction in the level of both endogenous and gamma-irradiation-induced p53 in HCT116 human colon carcinoma cells. This reduction was prevented by the proteasome inhibitors MG132 and lactacystin, suggesting enhanced p53 degradation in the presence of dicoumarol. Dicoumarol-induced degradation of p53 also was prevented in the presence of simian virus 40 large T antigen, which is known to bind and to stabilize p53. Cells overexpressing NQO1 were resistant to dicoumarol, and this finding indicates the direct involvement of NQO1 in p53 stabilization. NQO1 inhibition induced p53 degradation and blocked wild-type p53-mediated apoptosis in gamma-irradiated normal thymocytes and in M1 myeloid leukemic cells that overexpress wild-type p53. Dicoumarol also reduced the level of p53 in its mutant form in M1 cells. The results indicate that NQO1 plays an important role in regulating p53 functions by inhibiting its degradation.
Figures




References
-
- Oren M. FASEB J. 1992;6:3169–3176. - PubMed
-
- Levine A J. Cell. 1997;88:323–331. - PubMed
-
- Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Nature (London) 1991;352:345–347. - PubMed
-
- Lotem J, Sachs L. Blood. 1993;82:1092–1096. - PubMed
-
- Lowe S W, Schmitt E M, Smith S W, Osborne B A, Jacks T. Nature (London) 1993;362:847–849. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

