Prolonging the half-life of human interferon-alpha 2 in circulation: Design, preparation, and analysis of (2-sulfo-9-fluorenylmethoxycarbonyl)7- interferon-alpha 2
- PMID: 11158619
- PMCID: PMC14734
- DOI: 10.1073/pnas.98.3.1212
Prolonging the half-life of human interferon-alpha 2 in circulation: Design, preparation, and analysis of (2-sulfo-9-fluorenylmethoxycarbonyl)7- interferon-alpha 2
Abstract
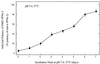
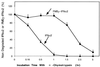
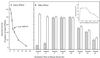
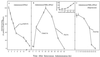
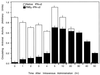
Polypeptide drugs are generally short-lived species in circulation. In this study, we have covalently linked seven moieties of 2-sulfo-9-fluorenylmethoxycarbonyl (FMS) to the amino groups of human interferon-alpha2. The derivative thus obtained (FMS(7)-IFN-alpha2) has approximately 4% the biological potency and 33 +/- 4% the receptor binding capacity of the native cytokine. Upon incubation, FMS(7)-IFN-alpha2 undergoes time-dependent spontaneous hydrolysis, generating active interferon with t(1/2) values of 24 +/- 2 h at pH 8.5 and 98 +/- 10 h at pH 7.4. When native IFN-alpha2 is intravenously administered to mice, circulating antiviral activity is maintained for a short duration and then declines with t(1/2) = 4 +/- 0.5 h, reaching undetectable values at approximately 18 h after administration. With intravenously administered FMS(7)-IFN-alpha2, there is a lag period of 2 h, followed by a progressive elevation in circulating antiviral-active protein, which peaked at 20 h and declined with t(1/2) = 35 +/- 4 h. FMS(7)-IFN-alpha2 is resistant to alpha-chymotrypsin digest and to proteolytic inactivation by human serum proteases in vitro. We have thus introduced here an inactive IFN-alpha2 derivative, which is resistant to in situ inactivation and has the capability of slowly reverting to the native active protein at physiological conditions in vivo and in vitro. Having these attributes, FMS(7)-IFN-alpha2 maintains prolonged circulating antiviral activity in mice, exceeding 7-8 times the activity of intravenously administered native cytokine.
Figures

References
-
- Goodman L, Gilman A E. In: Goodman & Gilman: Handbook of Pharmacology. Gilman A G, Rall T W, Nies A S, Taylor P, editors. Oxford: Pergamon; 1990.
-
- Olson T S, Dice J F. Curr Opin Cell Biol. 1989;1:1194–2000. - PubMed
-
- Barnes E, Webster G, Jacobs R, Dusheiko G. J Hepatol. 1999;31:244–249. - PubMed
-
- Lundgren E, Hedman H, Landberg G, Chiang F, Roos G, Sanders R. Eur J Cancer. 1991;27:S82–S84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

