Regulation of cystic fibrosis transmembrane conductance regulator single-channel gating by bivalent PDZ-domain-mediated interaction
- PMID: 11158634
- PMCID: PMC14749
- DOI: 10.1073/pnas.98.3.1300
Regulation of cystic fibrosis transmembrane conductance regulator single-channel gating by bivalent PDZ-domain-mediated interaction
Abstract
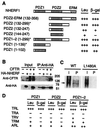
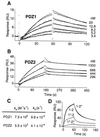
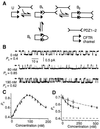
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-dependent protein kinase- and ATP-regulated chloride channel, the activity of which determines the rate of electrolyte and fluid transport in a variety of epithelial tissues. Here we describe a mechanism that regulates CFTR channel activity, which is mediated by PDZ domains, a family of conserved protein-interaction modules. The Na(+)/H(+) exchanger regulatory factor (NHERF) binds to the cytoplasmic tail of CFTR through either of its two PDZ (PDZ1 and PDZ2) domains. A recombinant fragment of NHERF (PDZ1-2) containing the two PDZ domains increases the open probability (P(o)) of single CFTR channels in excised membrane patches from a lung submucosal gland cell line. Both PDZ domains are required for this functional effect, because peptides containing mutations in either domain are unable to increase channel P(o). The concentration dependence of the regulation by the bivalent PDZ1-2 domain is biphasic, i.e., activating at lower concentrations and inhibiting at higher concentrations. Furthermore, either PDZ domain alone or together is without effect on P(o), but either domain can competitively inhibit the PDZ1-2-mediated stimulation of CFTR. Our results support a molecular model in which bivalent NHERF PDZ domains regulate channel gating by crosslinking the C-terminal tails in a single dimeric CFTR channel, and the magnitude of this regulation is coupled to the stoichiometry of these interactions.
Figures

Comment in
-
PDZ domains: More than just a glue.Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):787-9. doi: 10.1073/pnas.98.3.787. Proc Natl Acad Sci U S A. 2001. PMID: 11158544 Free PMC article. Review. No abstract available.
References
-
- Riordan J R, Rommens J M, Kerem B-S, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J-L, et al. Science. 1989;245:1066–1072. - PubMed
-
- Sheppard D N, Welsh M J. Physiol Rev. 1999;79:S23–S45. - PubMed
-
- Gadsby D C, Nairn A C. Physiol Rev. 1999;79:S77–S107. - PubMed
-
- Schwiebert E M, Benos D J, Egan M E, Stutts M J, Guggino W B. Physiol Rev. 1999;79:S145–S166. - PubMed
-
- Huang P, Trotter K, Boucher R C, Milgram S L, Stutts M J. Am J Physiol. 2000;278:C417–C422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

