Regulation of VanA- and VanB-type glycopeptide resistance in enterococci
- PMID: 11158729
- PMCID: PMC90301
- DOI: 10.1128/AAC.45.2.375-381.2001
Regulation of VanA- and VanB-type glycopeptide resistance in enterococci
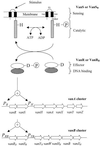
Figures

References
-
- Allen N E, Hobbs J N., Jr Induction of vancomycin resistance in Enterococcus faecium by non-glycopeptide antibiotics. FEMS Microbiol Lett. 1995;132:107–114. - PubMed
-
- Al-Obeid S, Billot-Klein D, Van Heijenoort J, Collatz E, Gutmann L. Replacement of the essential penicillin-binding protein 5 by high-molecular mass PBPs may explain vancomycin-β-lactam synergy in low-level vancomycin-resistant Enterococcus faecium D366. FEMS Microbiol Lett. 1992;91:79–84. - PubMed
-
- Arthur M, Depardieu F, Cabanie L, Reynolds P, Courvalin P. Requirement of the VanY and VanX d, d-peptidases for glycopeptide resistance in enterococci. Mol Microbiol. 1998;30:819–830. - PubMed
-
- Arthur M, Depardieu F, Courvalin P. Regulated interactions between partner and non-partner sensors and response regulators that control glycopeptide resistance gene expression in enterococci. Microbiology. 1999;145:1849–1858. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

