Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae
- PMID: 11158737
- PMCID: PMC90309
- DOI: 10.1128/AAC.45.2.433-438.2001
Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae
Abstract
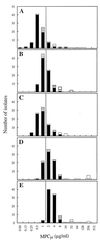
The mutant prevention concentration (MPC) represents a threshold above which the selective proliferation of resistant mutants is expected to occur only rarely. A provisional MPC (MPC(pr)) was defined and measured for five fluoroquinolones with clinical isolates of Streptococcus pneumoniae. Based on their potential for restricting the selection of resistant mutants, the five fluoroquinolones, in descending order, were found to be moxifloxacin > trovafloxacin > gatifloxacin > grepafloxacin > levofloxacin. For several compounds, 90% of about 90 clinical isolates that lacked a known resistance mutation had a value of MPC(pr) that was close to or below the serum levels that could be attained with a dosing regimen recommended by the manufacturers. Since MPC(pr) overestimates MPC, these data identify moxifloxacin and gatifloxacin as good candidates for determining whether MPC(pr) can be used as a guide for choosing and eventually administering fluoroquinolones to significantly reduce the development of resistance.
Figures
References
-
- Bingen E, Lambert-Zechovsky N, Mariani-Kurkdjian P, Doit C, Aujard Y, Fournerie F, Mathieu H. Bacterial counts in cerebrospinal fluid of children with meningitis. Eur J Clin Microbiol Infect Dis. 1990;9:278–281. - PubMed
-
- Blaser J, Stone B, Groner M, Zinner S. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987;31:1054–1060. - PMC - PubMed
-
- Blondeau J. A review of clinical trials with fluoroquinolones with an emphasis on new agents. Expert Opin Investig Drugs. 2000;9:383–413. - PubMed
-
- Chen D K, McGeer A, DeAzavedo J C, Low D E. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N Engl J Med. 1999;341:233–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

