Bioavailability and preliminary clinical efficacy of intrarectal artesunate in Ghanaian children with moderate malaria
- PMID: 11158748
- PMCID: PMC90320
- DOI: 10.1128/AAC.45.2.509-516.2001
Bioavailability and preliminary clinical efficacy of intrarectal artesunate in Ghanaian children with moderate malaria
Abstract
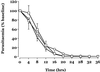
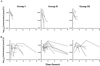
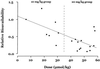
We report the first detailed pharmacokinetic assessment of intrarectal (i.r.) artesunate (ARS) in African children. Artesunate was given intravenously (i.v.; 2.4 mg/kg of body weight) and i.r. (10 or 20 mg/kg formulated as 50- or 200-mg suppositories [Rectocaps]) in a crossover study design to 34 Ghanaian children with moderate falciparum malaria. The median relative bioavailability of dihydroartemisinin (DHA), the active antimalarial metabolite of ARS, was higher in the low-dose i.r. group (10 mg/kg) than in the high-dose i.r. group (20 mg/kg) (58 versus 23%; P = 0.018). There was wide interpatient variation in the area under the concentration-time curve after i.r. ARS administration (up to 9-fold in the high-dose group and 20-fold in the low-dose group). i.r. administered ARS was more rapidly absorbed in the low-dose group than the high-dose group (median [range] absorption half-lives, 0.7 h [0.3 to 1.24 h] versus 1.1 h [0.6 to 2.7 h] [P = 0.023]. i.r. administered ARS was eliminated with a median (range) half-life of 0.8 h (0.4 to 2.7 h) (low-dose group and 0.9 h (0.1 to 2.5 h) (high-dose group) (P = 1). The fractional clearances of DHA were 3.9, 2.6, and 1.5 liters/kg/h for the 20-mg/kg, 10-mg/kg and i.v. groups, respectively (P = 0.001 and P = 0.06 for the high-and low-dose i.r. groups compared with the i.v. groups, respectively). The median volumes of distribution for DHA were 1.5 liters kg (20 mg/kg, i.r. group), 1.8 liters/kg (10 mg/kg, i.r. group), and 0.6 liters/kg (i.v. group) (P < 0.05 for both i.r. groups compared with the i.v. group). Parasite clearance kinetics were comparable in all treatment groups. i.r. administered ARS may be a useful alternative to parenterally administered ARS in the management of moderate childhood malaria and should be studied further.
Figures
References
-
- Agbenyega T, Angus B, Bedu-Addo G, Baffoe-Bonnie B, Guyton T, Stacpoole P, Krishna S. Glucose and lactate kinetics in children with severe malaria. J Clin Endocrinol Metab. 2000;85:1569–1576. - PubMed
-
- Barradell L B, Fitton A. Artesunate. A review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs. 1995;50:714–741. - PubMed
-
- Batty K T, Thu L T A, Ilett K E, Tien N P, Powell S M, Hung N C, Mai T X, Chon V V, Thien H V, Binh T Q, Kim N V, Davis T M E. A pharmacokinetic and pharmacodynamic study of artesunate for vivax malaria. Am J Trop Med Hyg. 1998;59:823–827. - PubMed
-
- Benakis A, Paris M, Loutan L, Plessas C T, Plessas S T. Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers. Am J Trop Med Hyg. 1997;56:17–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

