Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure
- PMID: 11159183
- PMCID: PMC1850286
- DOI: 10.1016/S0002-9440(10)63988-0
Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure
Abstract
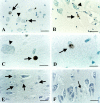
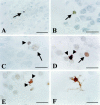
Glucocorticoid (GC) overexposure in animals has been implicated in hippocampal dysfunctioning and neuronal loss. In major depression, hypercortisolemia, hypothalamic-pituitary-adrenocortical-axis alterations, and reduced hippocampal volumes are commonly observed; hence, hippocampal neurodegeneration is also expected. To study possible GC-related pathology, we investigated hippocampal tissue of 15 major-depressed patients, 16 matched controls, and 9 steroid-treated patients, using in situ-end-labeling for DNA fragmentation and apoptosis, and heat-shock protein 70 and nuclear transcription factor kappaB immunocytochemistry for damage-related responses. No obvious massive cell loss was observed in any group. In 11 of 15 depressed patients, rare, but convincing apoptosis was found in entorhinal cortex, subiculum, dentate gyrus, CA1, and CA4. Also in three steroid-treated patients, apoptosis was found. Except for several steroid-treated patients, heat-shock protein 70 staining was generally absent, nor was nuclear transcription factor-kappaB activation found. The detection in 11 of 15 depressed patients, in three steroid-treated, and in one control patient, demonstrates for the first time that apoptosis is involved in steroid-related changes in the human hippocampus. However, in absence of major pyramidal loss, its rare occurrence, that notably was absent from areas at risk for GC damage such as CA3, indicates that apoptosis probably only contributes to a minor extent to the volume changes in depression.
Figures


References
-
- Raadsheer FC, Sluiter AA, Ravid R, Tilders FJ, Swaab DF: Localization of corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus of the human hypothalamus; age-dependent colocalization with vasopressin. Brain Res 1993, 615:50-62 - PubMed
-
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF: Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry 1995, 152:1372-1376 - PubMed
-
- Reul JM, de Kloet ER: Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 1985, 117:2505-2511 - PubMed
-
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M: Brain corticosteroid receptor balance in health and disease. Endocr Rev 1998, 19:269-301 - PubMed
-
- Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ: Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol 1995, 7:475-482 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

