PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification
- PMID: 11159191
- PMCID: PMC1850320
- DOI: 10.1016/S0002-9440(10)63996-X
PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification
Abstract
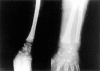
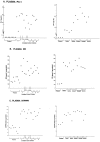
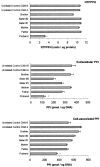
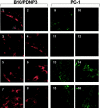
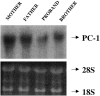
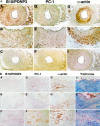
Inogranic pyrophosphate (PPi) inhibits hydroxyapatite deposition, and mice deficient in the PPi-generating nucleoside triphosphate pyrophosphohydrolase (NTPPPH) Plasma cell membrane glycoprotein-1 (PC-1) develop peri-articular and arterial calcification in early life. In idiopathic infantile arterial calcification (IIAC), hydroxyapatite deposition and smooth muscle cell (SMC) proliferation occur, sometimes associated with peri-articular calcification. Thus, we assessed PC-1 expression and PPi metabolism in a 25-month-old boy with IIAC and peri-articular calcifications. Plasma PC-1 was <1 ng/ml by enzyme-linked immunosorbent assay in the proband, but 10 to 30 ng/ml in unaffected family members and controls. PC-1 functioned to raise extracellular PPi in cultured aortic SMCs. However, PC-1 was sparse in temporal artery lesion SMCs in the proband, unlike the case for SMCs in atherosclerotic carotid artery lesions of unrelated adults. Proband plasma and explant-cultured dermal fibroblast NTPPPH and PPi were markedly decreased. The proband was heterozygous at the PC-1 locus, and sizes of PC-1 mRNA and polypeptide, and the PC-1 mRNA-coding region sequence were normal in proband fibroblasts. However, immunoreactive PC-1 protein was relatively sparse in proband fibroblasts. In conclusion, deficient extracellular PPi and a deficiency of PC-1 NTPPPH activity can be associated with human infantile arterial and peri-articular calcification, and may help explain the sharing of certain phenotypic features between some IIAC patients and PC-1-deficient mice.
Figures


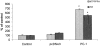
Similar articles
-
Up-regulated expression of the phosphodiesterase nucleotide pyrophosphatase family member PC-1 is a marker and pathogenic factor for knee meniscal cartilage matrix calcification.Arthritis Rheum. 2001 May;44(5):1071-81. doi: 10.1002/1529-0131(200105)44:5<1071::AID-ANR187>3.0.CO;2-3. Arthritis Rheum. 2001. PMID: 11352238
-
Differential mechanisms of inorganic pyrophosphate production by plasma cell membrane glycoprotein-1 and B10 in chondrocytes.Arthritis Rheum. 1999 Sep;42(9):1986-97. doi: 10.1002/1529-0131(199909)42:9<1986::AID-ANR26>3.0.CO;2-O. Arthritis Rheum. 1999. PMID: 10513816
-
Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein-1. Relevance to calcium pyrophosphate dihydrate deposition disease.Arthritis Rheum. 1994 Jun;37(6):934-41. doi: 10.1002/art.1780370624. Arthritis Rheum. 1994. PMID: 8003067
-
Inorganic pyrophosphate (PPI) in pathologic calcification of articular cartilage.Front Biosci. 2005 Jan 1;10:988-97. doi: 10.2741/1593. Print 2005 Jan 1. Front Biosci. 2005. PMID: 15569637 Review.
-
Animal models of pathologic calcification.Curr Opin Rheumatol. 2002 May;14(3):287-91. doi: 10.1097/00002281-200205000-00016. Curr Opin Rheumatol. 2002. PMID: 11981328 Review.
Cited by
-
Generalized Arterial Calcification of Infancy: Fatal Clinical Course Associated with a Novel Mutation in ENPP1.JIMD Rep. 2011;1:23-7. doi: 10.1007/8904_2011_11. Epub 2011 Jun 25. JIMD Rep. 2011. PMID: 23430823 Free PMC article.
-
Ultrastructure and biological function of matrix vesicles in bone mineralization.Histochem Cell Biol. 2018 Apr;149(4):289-304. doi: 10.1007/s00418-018-1646-0. Epub 2018 Feb 6. Histochem Cell Biol. 2018. PMID: 29411103 Review.
-
Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification.J Bone Miner Res. 2015 May;30(5):824-36. doi: 10.1002/jbmr.2420. J Bone Miner Res. 2015. PMID: 25428889 Free PMC article.
-
Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification.Kidney Int. 2008 May;73(9):1024-30. doi: 10.1038/ki.2008.26. Epub 2008 Feb 20. Kidney Int. 2008. PMID: 18288101 Free PMC article.
-
Phosphate: known and potential roles during development and regeneration of teeth and supporting structures.Birth Defects Res C Embryo Today. 2008 Dec;84(4):281-314. doi: 10.1002/bdrc.20136. Birth Defects Res C Embryo Today. 2008. PMID: 19067423 Free PMC article. Review.
References
-
- Schinke T, McKee MD, Kiviranta R, Karsenty G: Molecular determinants of arterial calcification. Ann Med 1998, 30:538-541 - PubMed
-
- Schinke T, McKee MD, Karsenty G: Extracellular matrix calcification: where is the action? Nat Genet 1999, 21:150-151 - PubMed
-
- Stary HC: Natural history of calcium deposits in atherosclerosis progression and regression. Zeitschrift fur Kardiologie 2000, 89(Suppl 2):S28-S35 - PubMed
-
- Bini A, Mann KG, Kudryk BJ, Schoen FJ: Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol 1999, 19:1852-1861 - PubMed
-
- Maayan C, Peleg O, Eyal F, Mogle P, Rosenmann E, Bar Ziv J: Idiopathic infantile arterial calcification: a case report and review of the literature. Eur J Pediatr 1984, 142:211-215 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

