Internalization of proteinase 3 is concomitant with endothelial cell apoptosis and internalization of myeloperoxidase with generation of intracellular oxidants
- PMID: 11159195
- PMCID: PMC1850298
- DOI: 10.1016/S0002-9440(10)64000-X
Internalization of proteinase 3 is concomitant with endothelial cell apoptosis and internalization of myeloperoxidase with generation of intracellular oxidants
Abstract
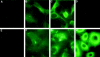
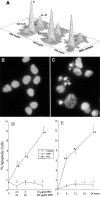
The important issue addressed by the studies presented here is the mechanism of neutrophil-mediated damage to endothelial and epithelial cells during inflammation. Binding of neutrophil-released granule proteins to endothelial cells may be involved in vascular damage in patients with inflammatory vascular diseases. We have determined whether granule proteins proteinase 3(PR3) and/or myeloperoxidase (MPO) are internalized into endothelial cells, as examined by UV light, confocal, and electron microscopy. Coincident induction of apoptosis and/or the generation of intracellular oxidants were monitored. The results indicate that human endothelial cells (human umbilical vein endothelial cells, human umbilical arterial endothelial cells, human lung microvascular endothelial cells) internalize both PR3 and MPO, which are detected on the cell surface, in the cytoplasm, and possibly nuclear. Epithelial cells (small airway epithelial cells) internalized MPO but not PR3, implying that the mechanism of PR3 internalization may be cell-type specific and different from that of MPO. Internalization of PR3, but not MPO, correlated with activation of apoptosis. Internalization of MPO correlated with an increase in intracellular oxidant radicals. The requirement for the proteolytic activity of PR3 for the induction of apoptosis was examined by generating PR3-truncated fragments that did not contain the components of the catalytic triad. An apoptotic function was localized to the C-terminal portion of PR3. These studies reveal novel mechanisms by which the neutrophil granule proteins PR3 and MPO contribute to tissue injury at sites of inflammation.
Figures




Similar articles
-
Proteinase 3-ANCA Vasculitis versus Myeloperoxidase-ANCA Vasculitis.J Am Soc Nephrol. 2015 Oct;26(10):2314-27. doi: 10.1681/ASN.2014090903. Epub 2015 May 8. J Am Soc Nephrol. 2015. PMID: 25956510 Free PMC article. Review.
-
Binding of proteinase 3 and myeloperoxidase to endothelial cells: ANCA-mediated endothelial damage through ADCC?Clin Exp Immunol. 1994 Jul;97(1):52-60. doi: 10.1111/j.1365-2249.1994.tb06579.x. Clin Exp Immunol. 1994. PMID: 8033421 Free PMC article.
-
ANCA antigens, proteinase 3 and myeloperoxidase, are not expressed in endothelial cells.Kidney Int. 2000 May;57(5):1981-90. doi: 10.1046/j.1523-1755.2000.00048.x. Kidney Int. 2000. PMID: 10792617
-
Neutrophil priming and apoptosis in anti-neutrophil cytoplasmic autoantibody-associated vasculitis.Kidney Int. 2001 May;59(5):1729-38. doi: 10.1046/j.1523-1755.2001.0590051729.x. Kidney Int. 2001. PMID: 11318943
-
Antiproteinase 3- and antimyeloperoxidase-associated vasculitis.Kidney Int. 2000 Jun;57(6):2195-206. doi: 10.1046/j.1523-1755.2000.00080.x. Kidney Int. 2000. PMID: 10844589 Review.
Cited by
-
Histone modification signature at myeloperoxidase and proteinase 3 in patients with anti-neutrophil cytoplasmic autoantibody-associated vasculitis.Clin Epigenetics. 2016 Aug 12;8:85. doi: 10.1186/s13148-016-0251-0. eCollection 2016. Clin Epigenetics. 2016. PMID: 27752292 Free PMC article.
-
Proteinase 3-ANCA Vasculitis versus Myeloperoxidase-ANCA Vasculitis.J Am Soc Nephrol. 2015 Oct;26(10):2314-27. doi: 10.1681/ASN.2014090903. Epub 2015 May 8. J Am Soc Nephrol. 2015. PMID: 25956510 Free PMC article. Review.
-
Inhibition of neutrophil-mediated production of reactive oxygen species (ROS) by endothelial cells is not impaired in anti-neutrophil cytoplasmic autoantibodies (ANCA)-associated vasculitis patients.Clin Exp Immunol. 2010 Aug;161(2):268-75. doi: 10.1111/j.1365-2249.2010.04171.x. Epub 2010 May 7. Clin Exp Immunol. 2010. PMID: 20456419 Free PMC article.
-
Mechanisms of vascular damage in ANCA vasculitis.Semin Immunopathol. 2022 May;44(3):325-345. doi: 10.1007/s00281-022-00920-0. Epub 2022 Mar 7. Semin Immunopathol. 2022. PMID: 35254509 Free PMC article. Review.
-
Pulmonary microvascular albumin leak is associated with endothelial cell death in murine sepsis-induced lung injury in vivo.PLoS One. 2014 Feb 7;9(2):e88501. doi: 10.1371/journal.pone.0088501. eCollection 2014. PLoS One. 2014. PMID: 24516666 Free PMC article.
References
-
- Dallegri F, Ottonello L: Tissue injury in neutrophilic inflammation. Inflamm Res 1997, 46:382-391 - PubMed
-
- Kunz M, Beutel S, Brocker EB: Leucocyte activation in erythema nodosum. Clin Exp Dermatol 1999, 24:396-401 - PubMed
-
- Owen CA, Campbell EJ: The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol 1999, 65:137-150 - PubMed
-
- Borregaard N, Cowland JB: Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89:3503-3521 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

