Neutrophil interaction with inflamed postcapillary venule endothelium alters annexin 1 expression
- PMID: 11159197
- PMCID: PMC1850304
- DOI: 10.1016/S0002-9440(10)64002-3
Neutrophil interaction with inflamed postcapillary venule endothelium alters annexin 1 expression
Abstract
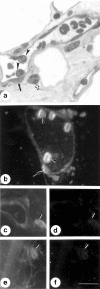
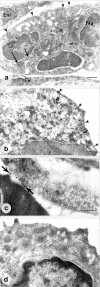
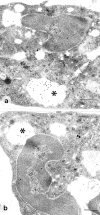
Annexin 1 (ANX-A1) exerts antimigratory actions in several models of acute and chronic inflammation. This is related to its ability to mimic the effect of endogenous ANX-A1 that is externalized on neutrophil adhesion to the postcapillary endothelium. In the present study we monitored ANX-A1 expression and localization in intravascular and emigrated neutrophils, using a classical model of rat peritonitis. For this purpose, a pair of antibodies raised against the ANX-A1 N-terminus (ie, able to recognize intact ANX-A1) or the whole protein (ie, able to interact with all ANX-A1 isoforms) was used by immunofluorescence and immunocytochemistry analyses. The majority ( approximately 50%) of ANX-A1 on the plasma membrane of intravascular neutrophils was intact. Extravasation into the subendothelial matrix caused loss of this pool of intact protein (to approximately 6%), concomitant with an increase in total amount of the protein; only approximately 25% of the total protein was now recognized by the antibody raised against the N-terminus (ie, it was intact). In the cytoplasm of these cells, ANX-A1 was predominantly associated with large vacuoles, possibly endosomes. In situ hybridization confirmed de novo synthesis of ANX-A1 in the extravasated cells. In conclusion, biochemical pathways leading to the externalization, proteolysis, and synthesis of ANX-A1 are activated during the process of neutrophil extravasation.
Figures



Similar articles
-
Cell localization of the anti-inflammatory protein annexin 1 during experimental inflammatory response.Ital J Anat Embryol. 2001;106(2 Suppl 1):69-77. Ital J Anat Embryol. 2001. PMID: 11729999
-
Annexin 1 localisation in tissue eosinophils as detected by electron microscopy.Mediators Inflamm. 2002 Oct;11(5):287-92. doi: 10.1080/09629350210000015683. Mediators Inflamm. 2002. PMID: 12467520 Free PMC article.
-
Antiallergic cromones inhibit neutrophil recruitment onto vascular endothelium via annexin-A1 mobilization.Arterioscler Thromb Vasc Biol. 2010 Sep;30(9):1718-24. doi: 10.1161/ATVBAHA.110.209536. Epub 2010 Jun 17. Arterioscler Thromb Vasc Biol. 2010. PMID: 20558817 Free PMC article.
-
Macrophage biology in the Anx-A1-/- mouse.Prostaglandins Leukot Essent Fatty Acids. 2005 Feb;72(2):95-103. doi: 10.1016/j.plefa.2004.10.008. Prostaglandins Leukot Essent Fatty Acids. 2005. PMID: 15626592 Review.
-
Cardioprotective potential of annexin-A1 mimetics in myocardial infarction.Pharmacol Ther. 2015 Apr;148:47-65. doi: 10.1016/j.pharmthera.2014.11.012. Epub 2014 Nov 25. Pharmacol Ther. 2015. PMID: 25460034 Review.
Cited by
-
Annexin A1 exerts renoprotective effects in experimental crescentic glomerulonephritis.Front Physiol. 2022 Oct 12;13:984362. doi: 10.3389/fphys.2022.984362. eCollection 2022. Front Physiol. 2022. PMID: 36311242 Free PMC article.
-
Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair.J Clin Invest. 2013 Jan;123(1):443-54. doi: 10.1172/JCI65831. Epub 2012 Dec 17. J Clin Invest. 2013. PMID: 23241962 Free PMC article.
-
Uneven modulation of the annexin 1 system in osteoblast-like cells by dexamethasone.Biochem Biophys Res Commun. 2007 Mar 9;354(2):414-9. doi: 10.1016/j.bbrc.2006.12.224. Epub 2007 Jan 10. Biochem Biophys Res Commun. 2007. PMID: 17254556 Free PMC article.
-
Transfection of annexin 1 in monocytic cells produces a high degree of spontaneous and stimulated apoptosis associated with caspase-3 activation.Br J Pharmacol. 2001 May;133(2):217-28. doi: 10.1038/sj.bjp.0704054. Br J Pharmacol. 2001. PMID: 11350857 Free PMC article.
-
Annexin A1 protein attenuates cyclosporine-induced renal hemodynamics changes and macrophage infiltration in rats.Inflamm Res. 2012 Mar;61(3):189-96. doi: 10.1007/s00011-011-0400-z. Epub 2011 Nov 19. Inflamm Res. 2012. PMID: 22101490
References
-
- Epstein FH: Tissue destruction by neutrophils. N Engl J Med 1989, 320:365-376 - PubMed
-
- Gallin JI, Goldstein IM, Snyderman R: Inflammation: Basic Principles and Clinical Correlates. 1992:pp 1-4 IM Goldstein. New York, Raven Press Ltd., Edited by JI Gallin
-
- Von Andrian UH, Hansell P, Chambers JD, Berger EM, Filho IT, Butcher EC, Arfors K-E: l-selectin function is required for β2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am J Physiol 1992, 263:H1034-H1044 - PubMed
-
- Hatanaka K, Katori M: Detachment of polymorphonuclear leukocytes adhered on venular endothelial cells. Microcirculation Ann 1992, 8:129-130
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

