Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review
- PMID: 11159655
- PMCID: PMC26574
- DOI: 10.1136/bmj.322.7282.329
Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review
Abstract
Objective: To assess the effectiveness and safety of antiplatelet drugs for prevention of pre-eclampsia and its consequences.
Design: Systematic review.
Data sources: Register of trials maintained by Cochrane Pregnancy and Childbirth Group, Cochrane Controlled Trials Register, and Embase.
Included studies: Randomised trials involving women at risk of pre-eclampsia, and its complications, allocated to antiplatelet drug(s) versus placebo or no antiplatelet drug.
Main outcome measures: Pre-eclampsia, preterm birth, fetal or neonatal death, and small for gestational age baby. Studies were assessed for quality of concealment of allocation and losses to follow up.
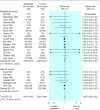
Results: 39 trials (30 563 women) were included, and 45 trials (>3000 women) excluded. Use of antiplatelet drugs was associated with a 15% reduction in the risk of pre-eclampsia (32 trials, 29 331 women; relative risk 0.85, 95% confidence interval 0.78 to 0.92; number needed to treat 100, 59 to 167). There was also an 8% reduction in the risk of preterm birth (23 trials, 28 268 women; 0.92, 0.88 to 0.97; 72, 44 to 200), and a 14% reduction in the risk of fetal or neonatal death (30 trials, 30 093 women; 0.86, 0.75 to 0.98; 250, 125 to >10 000) for women allocated antiplatelet drugs. Small for gestational age babies were reported in 25 trials (20 349 women), with no overall difference between the groups (relative risk 0.92, 0.84 to 1.01). There were no significant differences in other measures of outcome.
Conclusions: Antiplatelet drugs, largely low dose aspirin, have small to moderate benefits when used for prevention of pre-eclampsia.
Figures




Comment in
- ACP J Club. 2001 Jul-Aug;135(1):10
References
-
- Gifford RW, August P, Chesley LC, Cunningham G, Ferris TF, Lindheimer MD, et al. National high blood pressure education program working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 1990;163:1689–1712. - PubMed
-
- World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80–83. - PubMed
-
- Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99:547–553. - PubMed
-
- Department of Health; Welsh Office; Scottish Home and Health Department; Department of Health and Social Security. Report of confidential enquiries into maternal deaths in the United Kingdom 1994-1996. London: HMSO; 1998.
-
- Department of Health. Confidential enquiry into stillbirths and deaths in infancy: 3rd annual report. London: DoH; 1996.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
