Improved diabetic syndrome in C57BL/KsJ-db/db mice by oral administration of the Na(+)-glucose cotransporter inhibitor T-1095
- PMID: 11159708
- PMCID: PMC1572576
- DOI: 10.1038/sj.bjp.0703829
Improved diabetic syndrome in C57BL/KsJ-db/db mice by oral administration of the Na(+)-glucose cotransporter inhibitor T-1095
Abstract
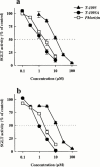
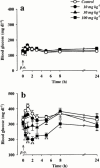
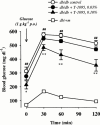
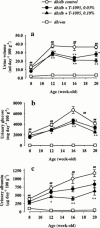
1. The therapeutic effects of an orally active inhibitor of Na(+)-glucose cotransporter (SGLT), T-1095 (a derivative of phlorizin; 3-(benzo[b]furan-5-yl)-2',6'-dihydroxy-4'-methylpropiophenone 2'-O-(6-O-methoxycarbonyl-beta-D-glycopyranoside)) were examined in C57BL/KsJ-db/db (db/db) mice, a genetic animal model of obese type 2 diabetes. 2. The higher renal SGLT activity in db/db mice than normoglycaemic C57BL/KsJ-db/+m (db/+m) mice may support the rationale for using an SGLT inhibitor in the treatment regimen for type 2 diabetes. Both T-1095 and its metabolite, T-1095A, which had approximately 10 times more potency, effectively inhibited renal SGLT activity of these mice in vitro. 3. Single oral administration of T-1095 (10, 30, 100 mg kg(-1), p.o.) to db/db mice caused a dose-dependent reduction in blood glucose levels and a concomitant increase in glucose excretion into urine. In contrast, T-1095 only slightly affected blood glucose levels in db/+m mice. 4. Chronic administration of T-1095 (0.1% w w(-1) pellet chow, for 12 weeks) decreased blood glucose and haemoglobin A(1C) levels, and improved glucose intolerance in db/db mice. The age-related decrease in plasma insulin levels was markedly inhibited and there was a 2.5 fold increase of insulin content in the pancreas of T-1095-treated db/db mice. Food consumption was not changed, while impaired body weight gain was ameliorated by T-1095 treatment. 5. Both the development of albuminuria and the expansion of glomerular mesangial area in db/db mice were significantly suppressed by chronic T-1095 treatment, indicating the prevention of the progression of diabetic nephropathy. 6. These results demonstrate that the SGLT inhibitor T-1095 is able to improve the metabolic abnormalities and inhibit the development of diabetic complications in db/db mice. Thus, T-1095 can be used for therapy of type 2 diabetic patients.
Figures


References
-
- BERGLUND O., FRANKEL B.J., HELLMAN B. Development of the insulin secretory defect in genetically diabetic (db/db) mouse. Acta Endocrinol. 1978;87:543–551. - PubMed
-
- BLONDEL O., BAILBE D., PORTHA B. Insulin resistance in rats with non-insulin-dependent diabetes induced by neonatal (5 days) streptozotocin: evidence for reversal following phlorizin treatment. Metabolism. 1990;39:787–793. - PubMed
-
- COHEN M.P., HUD E., WU V.Y. Amelioration of diabetic nephropathy by treatment with monoclonal antibodies against glycated albumin. Kidney Int. 1994;45:1673–1679. - PubMed
-
- THE DIABETES CONTROL AND COMPLICATIONS TRIAL (DCCT) RESEARCH GROUP Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720. - PubMed
-
- DEETJEN P., BAEYER H.V., DREXEL H.Renal handling of D-glucose and other sugars Textbook of Nephrology 1995Baltimore: Williams & Wilkins; 90–94.ed. Massry, S.G. & Glassock, R.J. pp
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

