Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens
- PMID: 11159715
- PMCID: PMC1572597
- DOI: 10.1038/sj.bjp.0703850
Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens
Abstract
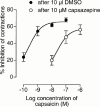
1. This study was directed at exploring the structure-activity relationship for anandamide and certain of its analogues at the rat VR1 receptor in transfected cells and at investigating the relative extent to which anandamide interacts with CB(1) and vanilloid receptors in the mouse vas deferens. 2. pK(i) values for displacement of [(3)H]-resiniferatoxin from membranes of rVR1 transfected CHO cells were significantly less for anandamide (5.78) than for its structural analogues N-(4-hydroxyphenyl)-arachidonylamide (AM404; 6.18) and N-(3-methoxy-4-hydroxy)benzyl-arachidonylamide (arvanil; 6.77). 3. pEC(50) values for stimulating (45)Ca(2+) uptake into rVR1 transfected CHO cells were significantly less for anandamide (5.80) than for AM404 (6.32) or arvanil (9.29). Arvanil was also significantly more potent than capsaicin (pEC(50)=7.37), a compound with the same substituted benzyl polar head group as arvanil. 4. In the mouse vas deferens, resiniferatoxin was 218 times more potent than capsaicin as an inhibitor of electrically-evoked contractions. Both drugs were antagonized to a similar extent by capsazepine (pK(B)=6.93 and 7.18 respectively) but were not antagonized by SR141716A (1 microM). Anandamide was less susceptible than capsaicin to antagonism by capsazepine (pK(B)=6.02) and less susceptible to antagonism by SR141716A (pK(B)=8.66) than methanandamide (pK(B)=9.56). WIN55212 was antagonized by SR141716A (pK(B)=9.02) but not by capsazepine (10 microM). 5. In conclusion, anandamide and certain of its analogues have affinity and efficacy at the rat VR1 receptor. In the mouse vas deferens, which seems to express vanilloid and CB(1) receptors, both receptor types appear to contribute to anandamide-induced inhibition of evoked contractions.
Figures



Similar articles
-
Evidence that the plant cannabinoid Delta9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist.Br J Pharmacol. 2005 Dec;146(7):917-26. doi: 10.1038/sj.bjp.0706414. Br J Pharmacol. 2005. PMID: 16205722 Free PMC article.
-
Arvanil-induced inhibition of spasticity and persistent pain: evidence for therapeutic sites of action different from the vanilloid VR1 receptor and cannabinoid CB(1)/CB(2) receptors.Eur J Pharmacol. 2002 Mar 29;439(1-3):83-92. doi: 10.1016/s0014-2999(02)01369-9. Eur J Pharmacol. 2002. PMID: 11937096
-
Regional differences in anandamide- and methanandamide-induced membrane potential changes in rat mesenteric arteries.J Pharmacol Exp Ther. 2001 Feb;296(2):322-8. J Pharmacol Exp Ther. 2001. PMID: 11160613
-
Anandamide: some like it hot.Trends Pharmacol Sci. 2001 Jul;22(7):346-9. doi: 10.1016/s0165-6147(00)01712-0. Trends Pharmacol Sci. 2001. PMID: 11431028 Review.
-
New perspectives on enigmatic vanilloid receptors.Trends Neurosci. 2000 Oct;23(10):491-7. doi: 10.1016/s0166-2236(00)01630-1. Trends Neurosci. 2000. PMID: 11006466 Review.
Cited by
-
Differential modulation of nociceptive versus non-nociceptive synapses by endocannabinoids.Mol Pain. 2013 Jun 1;9:26. doi: 10.1186/1744-8069-9-26. Mol Pain. 2013. PMID: 23725095 Free PMC article.
-
Biosynthesis of endocannabinoids and their modes of action in neurodegenerative diseases.Neurotox Res. 2003;5(3):183-200. doi: 10.1007/BF03033139. Neurotox Res. 2003. PMID: 12835123 Review.
-
Cannabinoid CB2 receptor-mediated anti-nociception in models of acute and chronic pain.Mol Neurobiol. 2007 Aug;36(1):26-35. doi: 10.1007/s12035-007-8007-7. Epub 2007 Oct 2. Mol Neurobiol. 2007. PMID: 17952647 Review.
-
Effects of WIN 55,212-2 on I(K) current in cultured trigeminal ganglion neurons of rat.J Huazhong Univ Sci Technolog Med Sci. 2005;25(2):124-6. doi: 10.1007/BF02873555. J Huazhong Univ Sci Technolog Med Sci. 2005. PMID: 16116951
-
Drug-induced mild therapeutic hypothermia obtained by administration of a transient receptor potential vanilloid type 1 agonist.BMC Cardiovasc Disord. 2010 Oct 9;10:51. doi: 10.1186/1471-2261-10-51. BMC Cardiovasc Disord. 2010. PMID: 20932337 Free PMC article.
References
-
- CHENG Y.-C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. - PubMed
-
- COLQUHOUN D. Lectures on Biostatistics. Oxford: Oxford University Press; 1971.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

