Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens
- PMID: 11159715
- PMCID: PMC1572597
- DOI: 10.1038/sj.bjp.0703850
Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens
Abstract
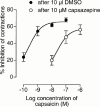
1. This study was directed at exploring the structure-activity relationship for anandamide and certain of its analogues at the rat VR1 receptor in transfected cells and at investigating the relative extent to which anandamide interacts with CB(1) and vanilloid receptors in the mouse vas deferens. 2. pK(i) values for displacement of [(3)H]-resiniferatoxin from membranes of rVR1 transfected CHO cells were significantly less for anandamide (5.78) than for its structural analogues N-(4-hydroxyphenyl)-arachidonylamide (AM404; 6.18) and N-(3-methoxy-4-hydroxy)benzyl-arachidonylamide (arvanil; 6.77). 3. pEC(50) values for stimulating (45)Ca(2+) uptake into rVR1 transfected CHO cells were significantly less for anandamide (5.80) than for AM404 (6.32) or arvanil (9.29). Arvanil was also significantly more potent than capsaicin (pEC(50)=7.37), a compound with the same substituted benzyl polar head group as arvanil. 4. In the mouse vas deferens, resiniferatoxin was 218 times more potent than capsaicin as an inhibitor of electrically-evoked contractions. Both drugs were antagonized to a similar extent by capsazepine (pK(B)=6.93 and 7.18 respectively) but were not antagonized by SR141716A (1 microM). Anandamide was less susceptible than capsaicin to antagonism by capsazepine (pK(B)=6.02) and less susceptible to antagonism by SR141716A (pK(B)=8.66) than methanandamide (pK(B)=9.56). WIN55212 was antagonized by SR141716A (pK(B)=9.02) but not by capsazepine (10 microM). 5. In conclusion, anandamide and certain of its analogues have affinity and efficacy at the rat VR1 receptor. In the mouse vas deferens, which seems to express vanilloid and CB(1) receptors, both receptor types appear to contribute to anandamide-induced inhibition of evoked contractions.
Figures



References
-
- CHENG Y.-C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. - PubMed
-
- COLQUHOUN D. Lectures on Biostatistics. Oxford: Oxford University Press; 1971.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

