Lipopolysaccharide-induced gelatinase granule mobilization primes neutrophils for activation by galectin-3 and formylmethionyl-Leu-Phe
- PMID: 11159975
- PMCID: PMC97959
- DOI: 10.1128/IAI.69.2.832-837.2001
Lipopolysaccharide-induced gelatinase granule mobilization primes neutrophils for activation by galectin-3 and formylmethionyl-Leu-Phe
Abstract
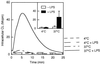
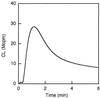
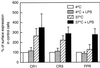
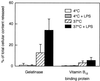
We have earlier shown that galectin-3, a lactose-binding mammalian lectin that is secreted from activated macrophages, basophils, and mast cells, induces activation of the NADPH oxidase in exudated but not in peripheral blood neutrophils (A. Karlsson, P. Follin, H. Leffler, and C. Dahlgren, Blood 91:3430-3438, 1998). The alteration in responsiveness occurring during extravasation correlated with mobilization of the gelatinase and/or specific granules to the cell surface, indicating a role for mobilizable galectin-3 receptors. In this study we have investigated galectin-3-induced NADPH oxidase activation, measured as superoxide production, in lipopolysaccharide (LPS)-primed neutrophils. Upon galectin-3 challenge, the LPS-primed cells produced superoxide, both extracellularly and intracellularly. A primed extracellular response to formylmethionyl-Leu-Phe (fMLF) was also achieved. The exposure of complement receptors 1 and 3 as well as the formyl peptide receptor on the cell surface was markedly increased after LPS treatment, indicating that granule fusion with the plasma membrane had occurred. Further assessment of specific markers for neutrophil granules showed that the LPS treatment had mobilized the gelatinase granules but only a minor fraction of the specific granules. We thus suggest that the mechanism behind LPS priming lies at the level of granule (receptor) mobilization for galectin-3 as well as for fMLF.
Figures


References
-
- Askew D, Yurochko A D, Burger C J, Elgert K D. Normal and tumor-bearing host macrophage responses: variability in accessory function, surface markers, and cell-cycle kinetics. Immunol Lett. 1990;24:21–29. - PubMed
-
- Babior B M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. - PubMed
-
- Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarström S, Holmes K V, Karlsson A, Kuroki M, Lin S H, Lucka L, Najjar S M, Neumaier M, Öbring B, Shively J E, Skubitz K M, Stanners C P, Thomas P, Thompson J A, Virji M, vonKleist S, Wagener C, Watt S, Zimmermann W. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–249. - PubMed
-
- Bennett T A, Lynam E B, Sklar L A, Rogelj S. Hydroxamate-based metalloprotease inhibitor blocks shedding of L-selectin adhesion molecule from leukocytes: functional consequences for neutrophil aggregation. J Immunol. 1996;156:3093–3097. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

